Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 1(1); 2010 > Article
-
Original Article
Gene Expression and Identification Related to Fluconazole Resistance ofCandida glabrata Strains - Jae Il Yoo1, Chi Won Choi2, Kyeong Min Lee1, Yeong Seon Lee1
-
Osong Public Health and Research Perspectives 2010;1(1):36-41.
DOI: https://doi.org/10.1016/j.phrp.2010.12.009
Published online: December 7, 2010
1Division of Antimicrobial Resistance, Center for Infectious Diseases, National Institute of Health, Korea Centers for Disease Control & Prevention, Seoul, Korea
2Proteome Research Team, Korea Basic Science Institute, Daejeon 3, Korea
- ∗Corresponding author. Division of Antimicrobial Resistance, Center for Infectious Diseases, National Institute of Health, Korea Centers for Disease Control & Prevention, Seoul, Korea. ysleenih@korea.kr
© 2010 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives
- Candida glabrata has become one of the most common causes of Candida bloodstream infections worldwide. Some strains of C. glabrata may be intermediately resistant to all azoles. The several possible mechanisms of azole resistance have been reported previously, but the exact resistant mechanism is not clear. In this study, we identified differentially expressed genes (DEGs) of fluconazole-resistant C. glabrata and compared the gene expression of fluconazole-resistant strains with that of fluconazole-susceptible strains to identify gene corresponding to fluconazole resistance.
-
Methods
- Using antifungal susceptibility test, several C. glabrata strains were selected and used for further study. The expression of CgCDR1 and CgCDR2 genes was investigated by slot hybridization against fluconazole-susceptible, -resistant, and resistant-induced strains. In addition, ERG3 and ERG11 genes were sequenced to analyze DNA base substitution. DEGs were identified by reverse transcription-polymerase chain reaction using DEG kit composed of 120 random primers.
-
Results
- In slot hybridization, CgCDR1 gene was expressed more than CgCDR2 gene in resistant strains. Though base substitution of ERG11 and ERG3 genes was observed in several base sequences, just one amino acid change was identified in resistant strain. In the results of reverse transcription-polymerase chain reaction, 44 genes were upregulated and 34 genes were downregulated. Among them, adenosine triphosphate-binding cassette transporter-related genes, fatty acid desaturase, lyase, and hypothetical protein genes were upregulated and aldehyde dehydrogenase, oxidoreductase, and prohibitin-like protein genes were downregulated. Other DEGs were also identified.
-
Conclusion
- This study showed that CgCDR1 gene was more closely related to fluconazole resistance of C. glabrata than CgCDR2 gene. In addition, several other genes related with fluconazole resistance of C. glabrata were identified.
- Since the early 1980s, fungi have emerged as major causes of human infectious diseases, especially among immunocompromised patients and those hospitalized with serious underlying disease.1–3 Candida is the agent most frequently implicated in invasive fungal infections, and it now ranks as the fourth most common cause of nosocomial bloodstream infections, accounting for 8% of all hospital-acquired bloodstream infections in the United States.4,5 Among intensive care unit patients, it is the third most common cause of nosocomial bloodstream infections.6 A recent trend noted in many hospitals is an increase in the prevalence of Candida glabrata as a cause of serious Candida infections.7,8 The rise in the number of C. glabrata systemic infections deserves a great deal of concern because of the high mortality rate associated with C. glabrata infection. The incidence of invasive candidiasis has increased worldwide in recent decades.9 Multiple antifungal agents are available for the treatment of candidiasis. Azole drugs, one of the four classes of antifungals, especially fluconazole, were commonly used in clinical practice. The extensive use of fluconazole has led to the increasing occurrence of resistant isolates. Especially, C. glabrata is yeast with intrinsically low susceptibility to fluconazole and is often recovered from clinical samples originating from AIDS or cancer patients.10 Four main mechanisms of azole resistance have been described:11 mutations in ERG11 gene; increases in the copy number of the azole target; the blockage of the ergosterol biosynthesis pathway; and the overexpression of genes coding some adenosine triphosphate (ATP)-binding cassette or major facilitator superfamily efflux pumps, leading to the increasing efflux of azole drugs. Although several possible mechanisms of azole resistance have been reported previously, the exact resistant mechanism is not clear. And there is little gene information about fluconazole resistance in C. glabrata. In this study, we investigated gene expression of fluconazole-resistant strains compared to susceptible strains to identify gene corresponding to fluconazole resistance. We used gene fishing primers, including 120 random primers, to discover gene for fluconazole resistance and identified gene.
Introduction
- 2.1 Clinical strains
- In this study, a total of 56 C. glabrata strains were collected from tertiary hospitals. The source of C. glabrata strains were blood, urine, sputum, and others. And just one strain was collected per patient. The collected strains were subcultured onto Sabouraud dextrose agar and CHROMagar Candida medium (CHROMagar Co., France) to ensure their viability and purity. Cultures were routinely inoculated from single colony and grown at 30°C in yeast extract peptone dextrose (YEPD) (10 g of yeast extract, 20 g of peptone, and 20 g of dextrose per liter) or on YEPD agar plates (10 g of yeast extract, 20 g of peptone, 20 g of dextrose, and 15 g of agar per liter), stored at 4°C, and subcultured weekly, or stored at −80°C in YEPD containing 10% glycerol. Using the source information and genetic background like restriction fragment length polymorphism, pulse-field gel electrophoresis pattern (data not shown), fluconazole-susceptible strain D-31 (minimal inhibitory concentration [MIC] 1 μg/mL), susceptible dose-dependent (SDD) strain D-284 (MIC 16 μg/mL), and resistant D-116 (MIC ≥256 μg/mL) strain were selected for further study.
- 2.2 Antifungal susceptibility testing
- Antifungal susceptibility testing of Candida strains to fluconazole was performed in exact accordance with the reference broth microdilution method described in the M27-A2 guidelines of the Clinical and Laboratory Standards Institute (formerly NCCLS).12
- 2.3 Fluconazole-resistant induction
- To investigate the expression signal change of CgCDR1 and CgCDR2 genes against fluconazole susceptibility step, fluconazole-susceptible D-31 strain was induced to be fluconazole resistant. C. glabrata D-31 strain was cultured in YEPD broth including fluconazole 1 μg/mL at the beginning, and the concentration of fluconazole was increased continuously up to 64 μg/mL. The MIC was confirmed with broth microdilution method and E-test experiment. The slot hybridization results were compared to results of susceptible, SDD, and resistant strains.
- 2.4 Expression of CgCDR1 and CgCDR2 genes
- The CgCDR1 and CgCDR2 genes were used for slot hybridization to analyse the patterns of gene expression of C. glabrata strain. Using polymerase chain reaction (PCR), CgCDR1, CgCDR2, and 26S ribosomal RNA products were synthesized, purified, and labelled by a random prime labelling kit (Amersham, Co., PA, USA). For hybridization experiment, nitrocellulose membrane was soaked in distilled water and in 20× saline-sodium citrate solution for 1 hour at room temperature. Sample RNA was added to the membrane and washed two to three times with 10× saline-sodium citrate solution, and the membrane was dried with a vacuum instrument. The RNA was fixed on the membrane with ultraviolet and hybridized. Signals were detected using an enhanced chemiluminescence detection kit (Amersham, Co., PA, USA). In slot hybridization experiment, fluconazole resistant-induced strains were also tested against CgCDR1 and CgCDR2 gene expression.
- 2.5 CgERG3 and CgERG11 sequence analysis
- Using three selected strains (fluconazole susceptible, SDD, resistant), ERG3 and ERG11 genes were detected by specific PCR with the use of ERG3-F, ERG3-R, ERG11-F, and ERG11-R primers complementary to the regions of ERG3 and ERG11 genes. The genomic DNA was used for PCR template. PCR ran in GeneAmp 2700 apparatus (Perkin-Elmer, Co., CA, USA) under the condition 25 cycles of PCR amplification with Taq DNA polymerase (Takara Shuzo Co., Shiga, Japan). Annealing reaction was done at 50°C, and extension reaction was done at 72°C. The product was confirmed with electrophoresis at 1.2% agarose gels. The PCR products of ERG3 and ERG11 genes were purified by PCR purification kit (Qiagene GmbH, Hilden, Germany) and directly sequenced.
- 2.6 Gene identification
- Cells from 24-hour cultures grown in YEPD were inoculated in 200 mL of YEPD at starting concentration of 2 × 104 cells/mL. The cultures were grown overnight at 30°C with agitation. Total RNAs were prepared from the cultures of susceptible, SDD, resistant strains at optical density of 600 nm of 0.3 value using RNeasy mini kit (Qiagen GmbH, Hilden, Germany). Total RNAs extracted from fluconazole-susceptible, -SDD, and -resistant strains were used for the synthesis of first-strand complementary DNAs (cDNAs) by reverse transcriptase. Reverse transcription was performed for 1.5 hours at 42°C in a final reaction volume of 20 μL containing 3 μg of the purified total RNA, 4 μL of 5′ reaction buffer (Promega, Madison, WI, USA), 5 μL of deoxynucleotide triphosphates (each 2 mM), 2 μL of 10-μM deoxythiamine annealing control primer 1(ACP-1) (5′-CTGTGAATGCTGCGACTAC GATIIIIIT-3′), 0.5 μL of RNasinÒ RNase inhibitor (40 U/μL; Promega), and 1 μL of Moloney murine leukaemia virus reverse transcriptase (200 U/μL; Promega). First-strand cDNAs were diluted by the addition of 80 μL of ultra-purified water for GeneFishing PCR and stored at −20°C until use. Differentially expressed genes were screened by the ACP-based PCR method using GeneFishing differentially expressed gene kits (Seegene, Seoul, South Korea). Briefly, second-strand cDNA synthesis was conducted at 50°C during one cycle of first-stage PCR in a final reaction volume of 20 μL containing 3–5 μL (about 50 ng) of diluted first-strand cDNA, 1 μL of dT-ACP2 (10 μM), 1 μL of 10 μM arbitrary ACP, and 10 μL of 2′ Master Mix (Seegene). The PCR protocol for second-strand synthesis was one cycle at 94°C for 1 minute, followed by 50°C for 3 minutes, and 72°C for 1 minute. After second-strand DNA synthesis was completed, the second-stage PCR amplification protocol was 40 cycles of 94°C for 40 seconds, 65°C for 40 seconds, and 72°C for 40 seconds, followed by a 5-minute final extension at 72°C. The amplified PCR products were separated in 2% agarose gel and stained with ethidium bromide. The differentially expressed bands were re-amplified and extracted from the gel using GENCLEANÒ II Kit (Q-BIO gene, Carlsbad, CA, USA) and directly sequenced with ABI PRISMÒ 3100-Avant Genetic Analyzer (Applied Biosystems, Foster City, CA, USA).
Materials and Methods
- 3.1 Expression of CgCDR1 and CgCDR2 genes
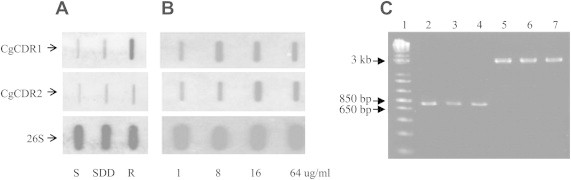
- In the results of hybridization experiments, the fluconazole-resistant D-116 strain showed increased expression of CgCDR1 gene than the susceptible (D-31) and SDD (D-284) strains. CgCDR2 gene expression also increased in the fluconazole-resistant strain than in the susceptible strain, but the signal was weaker than the CgCDR1 gene signal (Figure 1A). The fluconazole resistant-induced strain showed increased expression of CgCDR1 and CgCDR2 compared to susceptible strain. But CgCDR1 gene responded first to the fluconazole resistance induction environment (Figure 1B).
- 3.2 CgERG3 and CgERG11 sequence analysis
- Three C. glabrata strains showed the same predicted product size against ERG3 and ERG11 genes (Figure 1C). Three point mutations of ERG3 gene were observed in T435C, T317A, and G423A. In addition, two amino acid changes were also observed (Leu106Gln, Glu139Ala) including one in the fluconazole-resistant strain. Moreover, five point mutations were shown in ERG11 gene but no amino acid change (Table 1).
- 3.3 Gene identification
- When 120 random primers were used, the expression of 258 genes increased or decreased (Figure 2). Among these genes, 78 genes showed more than two times expression difference between fluconazole-susceptible and -resistant strains. Finally, 44 genes were of higher expression and 34 were of lower expression in the fluconazole-resistant strain than in the susceptible strain. Among the 44 genes, 13 were identified as fumarate hydratase, manganese transporter, transcriptional regulation mediator, adenosine diphosphate/ATP carrier protein, and other proteins. All of the proteins were corresponding to Saccharomyces cerevisiae, Saccharomyces kluyveri, and C. glabrata protein. The 34 less expressed genes included aldehyde dehydrogenase, oxidoreductase, and prohibitin-like protein gene. The ATP-binding cassette transporter was identified in both patterns (Table 2).
Results
- Candida species is one of the major organisms of fungal infections. Recently, C. glabrata has emerged as a common cause of bloodstream and mucosal infections. The major mechanisms of fluconazole resistance among C. glabrata described to date have been primarily based on CgCDR1, CgCDR2, and ergosterol synthesis pathway enzymes. The CgCDR1 and CgCDR2 gene upregulation and the point mutation of ERG3 and ERG11 genes are known to have related with fluconazole resistance of C. glabrata. However, there is not much information on other genes contributing to the fluconazole resistance of C. glabrata. In this study, we discovered that 258 genes were expressed differentially between fluconazole-susceptible and -resistant strains. Among them, 73 genes showed higher or lower expression in the resistant strain compared to the susceptible strain. Most of the identified genes were assigned to S cerevisiae because of insufficient information on C. glabrata gene even though the full sequence of C. glabrata was recently published.13 The identified gene products were corresponded to amino acid metabolism, cell wall synthesis, energy production protein, tricarboxylic acid cycle enzyme-related products, oxidoreductase, prohibitin-like protein, and others. Among the identified genes, specifically ATP-binding transporters increased or decreased. Because ATP-binding transporters like CgCDR1 and CgCDR2 genes showed higher expression in the fluconazole-resistant strain than in the susceptible strain, other ATP-binding transporters related to fluconazole resistance are of high interest. The expression of CgCDR1 has higher fluconazole-resistant levels than in the concentration of CgCDR2 expression.10 In our study, the hybridization experiment of CgCDR1 and CgCDR2 genes also showed that the expression of CgCDR1 gene was more increased than CgCDR2 compared to susceptible strain. And the expression of fluconazole resistant-induced strain showed that CgCDR1 gene was first increased to the fluconazole stress. This result supposes that CgCDR1 gene is the main gene corresponding to fluconazole resistance. And the point mutation of ERG3 gene was observed in three DNA bases. And it caused amino acid changes. The position of base substitution of ERG3 gene was different from the position previously reported.14–16 Usually, the point mutation of ERG3 or ERG11 gene is related with fluconazole resistance.17,18 But the results of this study need more sequence analysis using more fluconazole-susceptible strains to confirm the results. Finally, in this study, we identified a few genes whose expression increased or decreased in fluconazole-resistant strain. But the exact mechanism of how resistance is mediated has not been answered. Thus, it is necessary to study the functions of meaningful genes including transporter-related gene except already known CgCDR1 and CgCDR2 genes.
Discussion
-
Acknowledgements
- This study was supported by an intramural research grant of the Korea Centers for Disease Control and Prevention 2009.
Acknowledgement
-
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article information
- 1. Blumberg H.M., Jarvis W.R., Soucie J.M.. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin Infect Dis 33:2001;177−186. PMID: 11418877.ArticlePubMed
- 2. Pfaller M.A., Diekema D.J.. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20:2007;133−163. PMID: 17223626.ArticlePubMedPMC
- 3. Diekema D.J., Pfaller M.A.. Nosocomial candidemia: an ounce of prevention is better than a pound of cure. Infect Control Hosp Epidemiol 25:2004;624−626. PMID: 15357151.ArticlePubMed
- 4. Ghannoum M.A., Fu Y., Ibrahim A.S.. In vitro determination of optimal antifungal combinations against Cryptococcus neoformans and Candida albicans. Antimicrob Agents Chemother 39:1995;2459−2465. PMID: 8585726.ArticlePubMedPMC
- 5. Loeffler J., Stevens D.A.. Antifungal drug resistance. Clin Infect Dis 36:2003;S31−S41. PMID: 12516028.ArticlePubMed
- 6. Wisplinghoff H., Bischoff T., Tallent S.M.. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39:2004;309−317. PMID: 15306996.ArticlePubMed
- 7. Marr K.A., Seidel K., White T.C., Bowden R.A.. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J Infect Dis 181:2000;309−316. PMID: 10608780.ArticlePubMed
- 8. Malani P.N., Bradley S.F., Little R.S., Kauffman C.A.. Trends in species causing fungaemia in a tertiary care medical centre over 12 years. Mycoses 44:2001;446−449. PMID: 11820256.ArticlePubMed
- 9. Eggimann P., Garbino J., Pittet D.. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 3:2003;685−702. PMID: 14592598.ArticlePubMed
- 10. Sanglard D., Ischer F., Bille J.. Role of ATP-binding cassette transporter genes in high frequency acquisition of resistance to azole antifungals in Candida glabrata. Antimicrob Agents Chemother 45:2001;1174−1183. PMID: 11257032.ArticlePubMedPMC
- 11. Sanglard D.. Clinical relevance of mechanisms of antifungal drug resistance in yeasts. Enferm Infecc Microbiol Clin 20:2002;462−469. PMID: 12425880.ArticlePubMed
- 12. National Committee for Clinical Laboratory Standards . Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeast: Approved Standard M27-A2. 2002. NCCLS; Wayne, PA.
- 13. Dujon B., Sherman D., Fischer G.. Genome evolution in yeasts. Nature 430:2004;35−44. PMID: 15229592.ArticlePubMed
- 14. Lamb D.C., Kelly D.E., Schunck W.H.. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J Biol Chem 272:1997;5682−5688. PMID: 9038178.ArticlePubMed
- 15. Sanglard D., Ischer F., Koymans L., Bille J.. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother 42:1998;241−253. PMID: 9527767.ArticlePubMedPMCPDF
- 16. Vanden B.H., Marichal P., Gorrens J.. Mutation in cytochrome P-450-dependent 14 alpha-demethylase results in decreased affinity for azole antifungals. Biochem Soc Trans 18:1990;56−59. PMID: 2185088.ArticlePubMed
- 17. Sanguinetti M., Posteraro B., Fiori B.. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob Agents Chemother 49:2005;668−679. PMID: 15673750.ArticlePubMedPMC
- 18. Morschhauser J.. The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta 18:2002;240−248. PMID: 12084466.
References
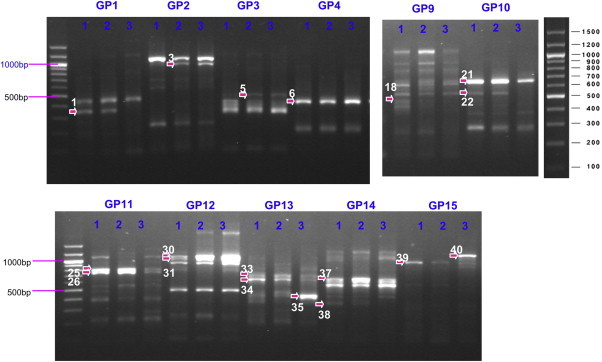
A total of 258 genes showed increased or decreased expression. Among them, 78 genes were selected for product cloning and sequencing.
tRNA = transfer RNA; cAMP = cyclic adenosine monophosphate; AAC2 ADP/ATP = adenosine diphosphate/adenosine triphosphate carrier 2; CoA = coenzyme A; GTP = guanosine triphosphate.
Figure & Data
References
Citations

- Molecular Mechanisms Associated with Antifungal Resistance in Pathogenic Candida Species
Karolina M. Czajka, Krishnan Venkataraman, Danielle Brabant-Kirwan, Stacey A. Santi, Chris Verschoor, Vasu D. Appanna, Ravi Singh, Deborah P. Saunders, Sujeenthar Tharmalingam
Cells.2023; 12(22): 2655. CrossRef - Candida glabrata: Pathogenicity and Resistance Mechanisms for Adaptation and Survival
Yahaya Hassan, Shu Yih Chew, Leslie Thian Lung Than
Journal of Fungi.2021; 7(8): 667. CrossRef - Candidiasis and Mechanisms of Antifungal Resistance
Somanon Bhattacharya, Sutthichai Sae-Tia, Bettina C. Fries
Antibiotics.2020; 9(6): 312. CrossRef -
A Transcriptomics Approach To Unveiling the Mechanisms of
In Vitro
Evolution towards Fluconazole Resistance of a
Candida glabrata
Clinical Isolate
Mafalda Cavalheiro, Catarina Costa, Ana Silva-Dias, Isabel M. Miranda, Can Wang, Pedro Pais, Sandra N. Pinto, Dalila Mil-Homens, Michiyo Sato-Okamoto, Azusa Takahashi-Nakaguchi, Raquel M. Silva, Nuno P. Mira, Arsénio M. Fialho, Hiroji Chibana, Acácio G. R
Antimicrobial Agents and Chemotherapy.2019;[Epub] CrossRef - Clonal Spread of Candida glabrata Bloodstream Isolates and Fluconazole Resistance Affected by Prolonged Exposure: a 12-Year Single-Center Study in Belgium
Berdieke Goemaere, Katrien Lagrou, Isabel Spriet, Marijke Hendrickx, Pierre Becker
Antimicrobial Agents and Chemotherapy.2018;[Epub] CrossRef - Candida antifungal drug resistance in sub-Saharan African populations: A systematic review
Charlene Wilma Joyce Africa, Pedro Miguel dos Santos Abrantes
F1000Research.2017; 5: 2832. CrossRef - Expression Patterns of ABC Transporter Genes in Fluconazole-Resistant Candida glabrata
Atefeh Abdollahi Gohar, Hamid Badali, Tahereh Shokohi, Mojtaba Nabili, Nasrin Amirrajab, Maryam Moazeni
Mycopathologia.2017; 182(3-4): 273. CrossRef - Glabridin induces overexpression of two major apoptotic genes, MCA1 and NUC1 , in Candida albicans
Mojtaba Nabili, Maryam Moazeni, Mohammad Taghi Hedayati, Parisa Aryamlo, Atefeh Abdollahi Gohar, Seyed Mehdi Madani, Hamed Fathi
Journal of Global Antimicrobial Resistance.2017; 11: 52. CrossRef - Candida antifungal drug resistance in sub-Saharan African populations: A systematic review
Charlene Wilma Joyce Africa, Pedro Miguel dos Santos Abrantes
F1000Research.2016; 5: 2832. CrossRef


 PubReader
PubReader Cite
Cite
