Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 8(5); 2017 > Article
-
Original Article
Characterization ofClostridium difficile Strains Isolated from Patients withC. difficile -associated Disease in Korea - Seung-Hak Choa, Jung-Whan Chonb, Kun-Ho Seob, Young Kwon Kimc, Jung-Beom Kimd, Young-Seok Bake, Woon-Won Jungf, Cheorl-Ho Kimg, Jong Tae Choih
-
Osong Public Health and Research Perspectives 2017;8(5):325-331.
DOI: https://doi.org/10.24171/j.phrp.2017.8.5.06
Published online: October 31, 2017
aDivision of Bacterial Disease Research, Center for Infectious Disease Research, Korea National Institute of Health, Cheongju, Korea
bKU Center for Food Safety, College of Veterinary Medicine, Konkuk University, Seoul, Korea
cDepartment of Biomedical Laboratory Science, College of Medical Sciences, Konyang University, Daejeon, Korea
dDepartment of Food Science and Technology, Sunchon National University, Suncheon, Korea
eDepartment of Emergency Medical Services, Sun Moon University, Asan, Korea
fDepartment of Biomedical Laboratory, Science College of Health Science, Cheongju University, Cheongju, Korea
gGlycobiology Unit, Department of Biological Science, Sungkyunkwan University and Samsung Advanced Institute for Health Science and Technology (SAIHST), Suwon, Korea
hDepartment of Biomedical Laboratory Science, Kyungdong University, Wonju, Korea
- Corresponding author: Jong Tae Choi, E-mail: choigo0244@naver.com
Copyright ©2017, Korea Centers for Disease Control and Prevention
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 4,176 Views
- 31 Download
- 1 Crossref
Abstract
-
Objectives
- Studies on Clostridium difficile are rare in Korea. We investigated the epidemiological characteristics of C. difficile isolates from patients with C. difficile-associated disease (CDAD) in Korea.
-
Methods
- Multiplex polymerase chain reaction was performed to detect the presence of tcdA and tcdB toxin genes. Antimicrobial susceptibility test was carried out by the disk-dilution method. C. difficile strains were subtyped by automated repetitive-element palindromic PCR (rep-PCR).
-
Results
- Among patients with CDAD, 73 (25.8%), 32 (11.3%), 32 (11.3%), and 26 (9.2%) suffered from pneumonia, cancer or neoplasm, diabetes, and colitis, respectively. Of all stool samples, 43 samples (15.2%) were positive for C. difficile strains. We observed two expression patterns of toxin genes: tcdA+/tcdB+ (86% isolates) and tcdA−/tcdB+ (14% isolates), with all isolates expressing tcdB. Furthermore, some isolates were resistant to clindamycin (65%), ampicillin (56%), and cefazolin (40%), but all were susceptible to vancomycin and metronidazole. The tested samples were classified into diverse clusters using automated rep-PCR.
-
Conclusion
- Our findings revealed the characteristics and antibiotic resistance of C. difficile isolates from patients in Korea. The epidemiological data may provide valuable insight into development of treatment strategies for C. difficile infections in Korea.
- Since 1978, Clostridium difficile has been known to cause pseudomembranous colitis (PMC) through the release of toxins, and infection with this pathogen is associated with antibiotic use in patients with diarrhea [1]. The two major C. difficile toxins include a 308 kDa enterotoxin (toxin A, tcdA) and 270 kDa cytotoxin (toxin B, tcdB), which constitute important pathogenic factors associated with colitis [2]. These toxins destroy the cytoskeleton of intestinal cells, inactivate proteins, damage cells and secretions of intestinal fluid, and induce inflammation and apoptosis. The symptoms associated with C. difficile infections (CDIs) are due to the expression of tcdA and tcdB genes. CDIs may be classified as mild with self-limiting diarrhea or severe with complications due to excessive infection [3]. Although the intake of excessive antibiotics is the main cause of infectious diarrhea, including CDI, CDI was also reported in low-risk groups of patients not using antibiotics. C. difficile-associated disease (CDAD) causes watery or mucous-containing stools accompanied with abdominal pain and fever [4]. The frequency of CDAD increases in low-risk groups and is related to functions associated with proton-pump inhibitors (PPIs) [5]. The activity of PPIs may increase the pH in the stomach, which may speed up C. difficile propagation in a repressed state [6,7].
- In recent years, the incidence of CDI has continuously increased in Europe and the United States and C. difficile is reported to be an important source of infection [8]. According to a domestic research literature related to CDI and PMC from 1988 to 1997 and single-agency data from 2000 to 2005, the frequency of infection during this period was not high [9]. However, CDAD incidence and severe illness in patients hospitalized between 2004 and 2008 was 1.7/1,000 and 2.7/1,000 people, respectively, as indicated in another study focusing on 17 university hospitals around Korea [10]. In this period, > 60% of 1,367 patients with CDAD were prescribed metronidazole during the initial treatment; only an 8.9% relapse rate and a 3.6% rate of severe illness and complications were reported. During this time, Korea exhibited fewer clinical manifestations of CDAD with better as compared with cases reported in Europe and the United States; however, the incidence rate continued to gradually increase [11]. Therefore, a survey of tcdA and tcdB genes from C. difficile isolated from patients with diarrhea and other diseases is required along with the epidemiological research comparing the information from these patients with the characteristics of C. difficile isolates. However, studies regarding C. difficile characteristics in patients with CDI in Korea are insufficient.
- In this study, we isolated C. difficile from patients and identified its toxin types, spore formation, and antibiotic susceptibility to compare and correlate these findings with other diseases found in the patient cohort. Moreover, we identified C. difficile lineages by performing repetitive element palindromic (rep)-polymerase chain reaction (PCR) and phylogenetic analyses on C. difficile strains isolated from patients admitted in a clinic in Korea.
INTRODUCTION
- 1. Collection of stool samples according to disease symptoms
- Stool samples were collected from 283 patients with diarrhea who were tested for CDI from April 2013 to October 2013 at a clinic in Korea. This study was approved by the Institutional Review Board of the Bundang Jesaeng General Hospital (LAB15-01). Medical records of the patients enrolled in this study were reviewed by performing an epidemiological analysis of age- and gender-specific patterns, duration of hospitalization, symptoms, and antibiotics used, to prevent patient duplication. Moreover, leukocyte number, total protein level, albumin level, bilirubin level, C-reactive protein (CRP) level, and erythrocyte sedimentation rate (ESR) were measured using blood assays.
- 2. Identification of bacterial isolates
- Stool samples were gently mixed with 95% (v/v) ethanol at 1:1 ratio and incubated for 1 hour at 25°C. The mixture was inoculated on ChromID C. difficile selective medium (bioMérieux, Marcy I’Etoile, France) and incubated under anaerobic conditions (Bugbox; Ruskinn Technology, Bridgend, UK) for 48 to 72 hours at 37°C. Black-colored colonies were inoculated on blood agar plates (BAPs) and incubated under anaerobic conditions for 48 to 72 hours at 37°C. An API rapid ID 32A system kit (bioMérieux) was used for C. difficile identification according to the manufacturer’s instructions.
- 3. PCR for toxin genes
- Isolated colonies grown on BAPs were analyzed by multiplex PCR for the presence of tcdA and tcdB. DNA was extracted using QIAamp DNA mini kit (51306; Qiagen, Hilden, Germany) according to the manufacturer’s instructions. PCR was performed using a PerkinElmer thermal cycler PCR system 2400 (PerkinElmer, Waltham, MA, USA). The primers used for the detection of tcdA and tcdB were as follows: tcdA forward, 5′-AGA TTC CTA TAT TTA CAT GAC AAT AT-3′; tcdA reverse, 5′-GTA TCA GGC ATA AAG TAA TAT ACT TT-3′; and tcdB forward, 5′-GGA AAA GAG AAT GGT TTT ATT AA-3′; tcdB reverse, 5′-ATC TTT AGT TAT AAC TTT GAC ATC TTT-3′. The reaction conditions were as follows: initial denaturation at 95°C for 15 minutes, 30 cycles of denaturation at 95°C for 1 minute, annealing at 55°C for 2 minutes, extension at 72°C for 2 minutes, and final extension at 72°C for 5 minutes. The PCR product was subjected to gel electrophoresis using 1% agarose, followed by staining with ethidium bromide and visualization under an ultraviolet trans-illuminator.
- 4. Microscopic analysis
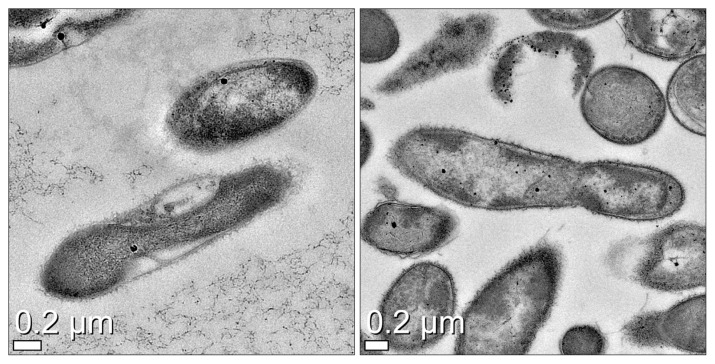
- For determining spores in the isolates, C. difficile strains positive for tcdA/B were directly examined by electron microscopy. The bacterial cells were diluted in 1.5 mL of 0.85% saline and washed thrice at 12,000 rpm. The bacterial sample was fixed in 2.5% glutaraldehyde/0.1 M phosphate-buffered saline (PBS; pH 7.4) for 2 hours and then transferred to 1% osmium tetroxide for 1 hour. The sample was washed with 0.1 M PBS, embedded into epoxy resin using propylene oxide, and dehydrated using alcohol. After embedding, the sample was polymerized in a dry oven at 60°C for 12 hours. In the subsequent steps, the bacteria-embedded resin was cut into 70 to 80 mm thick sections using an ultra-microtome (Ultra cut C; Leica, Wetzlar, Germany) and observed under an electron microscope (Cryo-TEM CryoTecnai F20; FEI, Hillsboro, OR, USA) after staining with uranyl acetate and lead citrate. Two standard strains, ATCC-43598 (tcdA−/tcdB+) and KCCH-12115 (tcdA+/tcdB+), were used for the comparison of spores.
- 5. Antimicrobial susceptibility
- The antimicrobial susceptibility of C. difficile isolates was evaluated by the disk-dilution method using ampicillin, cefazolin, imipenem, amikacin, clindamycin, and metronidazole. The E-test (bioMérieux) method for evaluating vancomycin resistance was also performed according to the guidelines of the Clinical and Laboratory Standards Institute.
- 6. Rep-PCR analysis
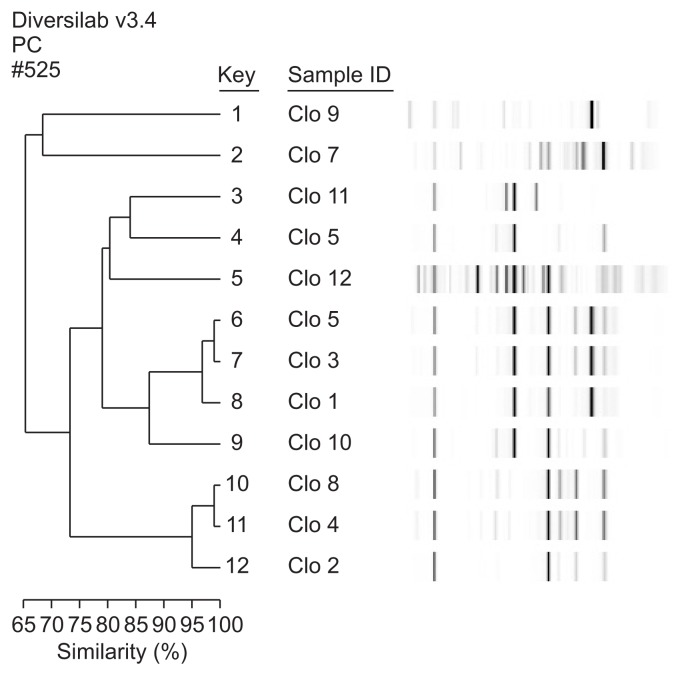
- Twelve randomly selected isolates, including two reference strains, were used for molecular subtyping. DNA was extracted from each strain using a commercially available DNA extraction kit (UltraClean microbial DNA isolation kit; MoBio Laboratories, Solana Beach, CA, USA) according to manufacturer’s instructions. Extracted DNA samples were quantified using a NanoDrop 2000 (Thermo Fisher Scientific, Wilmington, DE, USA), followed by their amplification for DNA fingerprinting. The PCR reaction contained 2 μL of extracted genomic DNA (~25 ng/μL) and 23 μL of PCR mixture containing 0.5 μL (or 2.5 U) AmpliTaq polymerase (Applied Biosystems, Foster City, CA, USA), 2.5 μL 10× GeneAMP PCR buffer I (Applied Biosystems), 2 μL primer mix, and 18 μL rep-PCR master mix from the DiversiLab Clostridium kit (bioMérieux). Amplification was performed under following conditions: initial denaturation at 94°C for 2 minutes, 35 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, extension at 70°C for 90 secodns, and final extension at 70°C for 3 minutes. PCR products were separated and analyzed using a microfluidic chip and an Agilent model 2100 bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). All tested samples were automatically analyzed by the Extended Jaccard coefficient using DiversiLabTM software (v2.1.66; bioMérieux). Dendrograms were created by determining the distance matrices and using the unweighted pair-group method with arithmetic mean. Banding patterns exhibiting high degrees of similarity (cut-off value, 98%) were considered indistinguishable.
MATERIALS AND METHODS
- 1. Patient profiles
- As shown in Table 1, there were more females than males (54.4% vs. 45.6%, respectively) among 283 patients enrolled. The average age was 64 ± 21 years; 66 patients (23.3%) aged between 70 and 79 years and 62 (22.9%) patients, between 80 and 89 years. The average duration of hospitalization was 30 ± 56 days and diarrhea occurrence was reported at 20.4 days after antibiotic administration. Among patients with CDAD, 73 (25.8%) also suffered from pneumonia, 32 (11.3%) from cancer or neoplasm, 32 (11.3%) from diabetes, and 26 (9.2%) from colitis (Table 2). The results of the hematological examination are shown in Table 3. In particular, parameters related to inflammation, such as white blood cell (WBC) count, ESR, and CRP level, were higher than other factors. A high percentage of patients used antibiotics (92.9% [263/283 patients]); 88 patients were administered with cephalosporin series of antibiotics (33.4%), while 121 patients were treated with glycopeptides series of antibiotics (vancomycin, 67 [25.5%] and carbapenem, 54 [20.5%]; Table 4). Many patients used several series of antibiotics together; 22 patients used four series and 21 patients, three series.
- 2. Toxin gene patterns, spore determination, and antibiotic susceptibility of C. difficile isolates
- Among 283 stool samples tested using ChromID C. difficile selective media, 43 samples (15.2%) were positive for C. difficile strains. In addition, two subtypes of C. clostridioforme—one subtype of C. septicum and three subtypes of C. perfringens—were isolated. The toxin genes (tcdA and tcdB) in C. difficile isolates were detected using PCR amplification and all isolates tested in this study showed the expression of tcdB; only 37 of 43 samples were detected positive for tcdA gene. We found two major expression patterns for the toxin genes: tcdA+/tcdB+ (37 C. difficile isolates; 86%) and tcdA−/tcdB+ (6 isolates; 14%). Electron microscopy results revealed that the spores of most C. difficile isolates were in their original form (Figure 1). As shown in Table 5, some isolates showed high resistance to clindamycin (65%), ampicillin (56%), and cefazolin (40%) but all were susceptible to vancomycin and metronidazole.
- 3. Rep-PCR analysis of C. difficile isolates
- Ten representative C. difficile strains isolated from patients with different diseases and two reference strains were subtyped by automated rep-PCR. The dendrogram and computer-generated banding-pattern images are presented in Figure 2. All tested isolates were classified into various clusters with similarities ranging from 65.8% to 99.5%. With the exception of two strains (number 6 and 7), all tested C. difficile isolates appeared to be genetically unrelated. Most isolates carrying similar characteristics such as antibiotic resistance and toxin profiles exhibited distinguishable rep-PCR banding patterns, suggesting that automated rep-PCR successfully discriminated C. difficile strains isolated from the patients. Two strains (number 6 and 7) displayed identical banding patterns, with over 99% similarity.
RESULTS
- C. difficile is an important bacterium that causes healthcare-associated infections (HAIs); therefore, it is important to report the sources of the disease for accurate diagnosis, given that C. difficile toxin causes diarrhea and PMC associated with antibiotic use [12]. CDI may be classified as mild (with self-limiting diarrhea and may not require treatment), moderate, or severe (with complications). In particular, severe CDI is defined as a dangerous infection characterized by an increase in WBC count (> 15,000/mm3) and in serum creatinine level (> 1.5-fold), and it may exhibit various complications such as lower blood pressure, shock, sepsis, intestinal paralysis, megacolon, gastrointestinal perforation, and death [3]. In Korea, the use of antibiotics and PPIs continues to increase, owing to inadequate restrictions related to the antibiotic use. A high proportion of patients (92.9%) in this study cohort was treated with antibiotics, and several antibiotic series were used together for treating many patients. The study cohort also included a higher proportion of older patients (aged 70–89 years) with weak immune systems. Hence, HAI caused by C. difficile are expected to be more frequent in Korea. As a result, there is an increased interest in the diagnosis of CDI. A previous study reported that rapid and accurate C. difficile diagnosis is critical for the treatment of patients and prevention of antibiotic abuse [13].
- The expression pattern of toxin genes (tcdA and tcdB) in C. difficile isolates was studied by PCR amplification. tcdA+/tcdB+ pattern constituted a major subtype (86%); however, there exists a gradually increasing trend of C. difficile isolates in Korea exhibiting a tcdA−/tcdB+ subtype. A study comparing the expression pattern of C. difficile tcdA/B toxins in isolates from CDI patients reported that tcdA−/tcdB+ subtype constituted 7% of subtypes detected in 2002 but rose to 27.0% by 2005 [13]. Toxin B is 1,000-fold more toxic than toxin A [14]. tcdA−/tcdB+ subtype is the result of a mutation in the repeat sequence of tcdA gene [2]. Hyper-virulent strains related to severe CDI are called as restriction endonuclease-analysis group B1/North American pulsed-field gel electrophoresis type 1 (NAP1)/ribotype 027 (B1/NAP1/ribotype 027). A change in the sequence of the tcdC repressor gene results in the loss of its inhibitory function against the transcription of tcdA and tcdB in the virulent B1/NAP1/ribotype 027 strain. Therefore, this strain produces 16-fold higher concentrations of toxin A and 23-fold higher concentrations of toxin B, which results in the resistance to fluoroquinolone series of antibiotics [15]. Furthermore, this strain produces a third toxin called binary toxin (actin-specific ADP-ribosyltransferase), which exhibits synergistic effects with toxins A and B, thereby leading to more severe CDIs [16]. The occurrence of recurrent stomach PMC in patients infected with strain BI/NAP1/ribotype 027 is suggestive of the increase in its repetition rate [15]. In this study, tcdB was detected in all isolates. The toxigenic properties of C. difficile strains isolated from patients with CDAD in Korea have continued to increase, as previously reported [13]. Therefore, it is essential to examine toxin B (encoded by tcdB) along with toxin A (encoded by tcdA) for the accurate detection of C. difficile and diagnosis of CDI.
- C. difficile is among the major bacteria associated with HAIs and the infection is known to be transmitted by spores in most hospitals [12]. All C. difficile isolates tested in this study showed spore formation; therefore, care must be taken to prevent infections in hospitals. CDI is classified as moderate or severe with complications, and metronidazole and vancomycin have been used as primary drugs for CDI treatment [14]. Results of the antibiotic susceptibility test showed that all isolates were susceptible to vancomycin and metronidazole. Rep-PCR is a very powerful tool used for the determination of bacterial lineages [17]. Here, we used rep-PCR to characterize C. difficile lineages based on the correlation between the information from patients and C. difficile isolates. We observed that the differences in the banding patterns in rep-PCR were associated with different diseases in patients. Two C. difficile isolates (number 6 and 7) exhibited > 99% similarity in their banding patterns. Although these two isolates were obtained from different patients, these two patients were hospitalized in the same ward and at the same time, indicating that these genetically identical strains may have originated from HAI. Conversely, most tested isolates showed distinguishable banding patterns, indicating that automated rep-PCR may be considered as an effective subtyping tool for tracking the source of infections (e.g., hospital- or community-acquired CDAD). In the case of CDI, prevention is more important than treatment. Development of vaccines against C. difficile was previously attempted [18]; however, no effective vaccine has been developed yet.
- In conclusion, our study on the characterization of C. difficile provided useful information for developing strategies to prevent CDI, especially hospital- and community-acquired CDAD. These results contribute to the accurate determination of suitable antibiotic treatment and drug development against C. difficile.
DISCUSSION
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
- 1. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 2009;7:526−36. https://doi.org/10.1038/nrmicro2164. PMID: 10.1038/nrmicro2164. PMID: 19528959.ArticlePubMed
- 2. Delmée M, Van Broeck J, Simon A, et al. Laboratory diagnosis of Clostridium difficile-associated diarrhoea: a plea for culture. J Med Microbiol 2005;54:187−91. https://doi.org/10.1099/jmm.0.45844-0. PMID: 10.1099/jmm.0.45844-0. PMID: 15673515.ArticlePubMed
- 3. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010;31:431−55. https://doi.org/10.1086/651706. PMID: 10.1086/651706. PMID: 20307191.ArticlePubMed
- 4. Cho HJ, Ryoo E, Sun YH, et al. Epidemiology and clinical characteristics of Clostridium difficile-associated diarrhea in children: comparison between community- and hospital-acquired infections. Korean J Pediatr Gastroenterol Nutr 2010;13:146−53. https://doi.org/10.5223/kjpgn.2010.13.2.146. PMID: 10.5223/kjpgn.2010.13.2.146.Article
- 5. Byun TJ, Han DS, Ahn SB, et al. Clinical characteristics and changing epidemiology of Clostridium difficile-associated disease (CDAD). Korean J Gastroenterol 2009;54:13−9. In Korean. https://doi.org/10.4166/kjg.2009.54.1.13. PMID: 10.4166/kjg.2009.54.1.13. PMID: 19696545.
- 6. Aseeri M, Schroeder T, Kramer J, et al. Gastric acid suppression by proton pump inhibitors as a risk factor for clostridium difficile-associated diarrhea in hospitalized patients. Am J Gastroenterol 2008;103:2308−13. https://doi.org/10.1111/j.1572-0241.2008.01975.x. PMID: 10.1111/j.1572-0241.2008.01975.x. PMID: 18702653.ArticlePubMed
- 7. Kazakova SV, Ware K, Baughman B, et al. A hospital outbreak of diarrhea due to an emerging epidemic strain of Clostridium difficile. Arch Intern Med 2006;166:2518−24. https://doi.org/10.1001/archinte.166.22.2518. PMID: 10.1001/archinte.166.22.2518. PMID: 17159019.ArticlePubMed
- 8. Ricciardi R, Rothenberger DA, Mandoff RD, et al. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg 2007;142:624−31. discussion 631. https://doi.org/10.1001/archsurg.142.7.624. PMID: 10.1001/archsurg.142.7.624. PMID: 17638799.ArticlePubMed
- 9. Lee JH, Lee SY, Kim YS, et al. The incidence and clinical features of Clostridium difficile infection; single center study. Korean J Gastroenterol 2010;55:175−82. In Korean. https://doi.org/10.4166/kjg.2010.55.3.175. PMID: 10.4166/kjg.2010.55.3.175. PMID: 20357528.
- 10. Kim HJ, Jeong SH, Roh KH, et al. Investigation of toxin gene diversity, molecular epidemiology, and antimicrobial resistance of Clostridium difficile isolated from 12 hospitals in South Korea. Korean J Lab Med 2010;30:491−7. https://doi.org/10.3343/kjlm.2010.30.5.491. PMID: 10.3343/kjlm.2010.30.5.491. PMID: 20890081.ArticlePubMed
- 11. Lee YJ, Choi MG, Lim CH, et al. Change of Clostridium difficile colitis during recent 10 years in Korea. Korean J Gastroenterol 2010;55:169−74. In Korean. https://doi.org/10.4166/kjg.2010.55.3.169. PMID: 10.4166/kjg.2010.55.3.169. PMID: 20357527.
- 12. Kim J, Pai H, Seo MR, et al. Epidemiology and clinical characteristics of Clostridium difficile infection in a Korean tertiary hospital. J Korean Med Sci 2011;26:1258−64. https://doi.org/10.3346/jkms.2011.26.10.1258. PMID: 10.3346/jkms.2011.26.10.1258. PMID: 22022175.ArticlePubMedPMC
- 13. Shin BM, Kuak EY, Yoo HM, et al. Multicentre study of the prevalence of toxigenic Clostridium difficile in Korea: results of a retrospective study 2000–2005. J Med Microbiol 2008;57:697−701. https://doi.org/10.1099/jmm.0.47771-0. PMID: 10.1099/jmm.0.47771-0. PMID: 18480325.ArticlePubMed
- 14. Kim SW. Treatment of refractory or recurrent Clostridium difficile infection. Korean J Gastroenterol 2012;60:71−8. https://doi.org/10.4166/kjg.2012.60.2.71. PMID: 10.4166/kjg.2012.60.2.71. PMID: 22926117.ArticlePubMed
- 15. Barbut F, Braun M, Burghoffer B, et al. Rapid detection of toxigenic strains of Clostridium difficile in diarrheal stools by real-time PCR. J Clin Microbiol 2009;47:1276−7. https://doi.org/10.1128/JCM.00309-09. PMID: 10.1128/JCM.00309-09. PMID: 19244461.ArticlePubMedPMC
- 16. Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet 2005;366:1079−84. https://doi.org/10.1016/S0140-6736(05)67420-X. PMID: 10.1016/S0140-6736(05)67420-X. PMID: 16182895.ArticlePubMed
- 17. Chon JW, Kim JH, Lee SJ, et al. Toxin profile, antibiotic resistance, and phenotypic and molecular characterization of Bacillus cereus in Sunsik. Food Microbiol 2012;32:217−22. https://doi.org/10.1016/j.fm.2012.06.003. PMID: 10.1016/j.fm.2012.06.003. PMID: 22850397.ArticlePubMed
- 18. Huang H, Weintraub A, Fang H, et al. Comparison of a commercial multiplex real-time PCR to the cell cytotoxicity neutralization assay for diagnosis of clostridium difficile infections. J Clin Microbiol 2009;47:3729−31. https://doi.org/10.1128/JCM.01280-09. PMID: 10.1128/JCM.01280-09. PMID: 19741082.ArticlePubMedPMC
REFERENCES
| Antibiotic | Number of cases (%) |
|---|---|
| Cephalosporin | 88 (33.4) |
| Glycopeptides (vancomycin)a | 67 (25.5) |
| Carbapenemb | 54 (20.4) |
| Penicillinb | 23 (8.7) |
| Glycopeptides (teicoplanin)b | 18 (6.7) |
| Amphotericin Bb | 5 (2.1) |
| Colistinb | 4 (1.6) |
| Linezolidb | 3 (1.2) |
| Metronidazolea | 1 (0.4) |
Figure & Data
References
Citations

- Metronidazole therapy as initial treatment of Clostridium difficile infection in patients with chronic kidney disease in Korea
Jaeuk Shin, Yu Mi Wi, Yu-Ji Lee
Epidemiology and Infection.2019;[Epub] CrossRef


 PubReader
PubReader Cite
Cite
