Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 8(6); 2017 > Article
-
Brief Report
In Vitro Antiviral Activity of Sakuranetin against Human Rhinovirus 3 - Hwa-Jung Choi
-
Osong Public Health and Research Perspectives 2017;8(6):415-420.
DOI: https://doi.org/10.24171/j.phrp.2017.8.6.09
Published online: December 31, 2017
Department of Beauty Science, Kwangju Women’s University, Gwangju, Korea
- Corresponding author: Hwa-Jung Choi, E-mail: rerived@empal.com
• Received: May 13, 2017 • Revised: September 22, 2017 • Accepted: October 9, 2017
Copyright ©2017, Korea Centers for Disease Control and Prevention
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- Rhinoviruses (RVs) cause common cold and are associated with exacerbation of chronic inflammatory respiratory diseases. Until now, no clinically effective antiviral chemotherapeutic agents to treat diseases caused by human rhinoviruses (HRVs) have been reported. We assessed the anti-HRV3 activity of sakuranetin isolated from Sorbus commixta Hedl. in human epithelioid carcinoma cervix (HeLa) cells, to evaluate its anti-rhinoviral potential in the clinical setting.
-
Methods
- Antiviral activity and cytotoxicity as well as the effect of sakuranetin on HRV3-induced cytopathic effects (CPEs) were evaluated using the sulforhodamine B (SRB) method using CPE reduction. The morphology of HRV3-infected cells was studied using a light microscope.
-
Results
- Sakuranetin actively inhibited HRV3 replication and exhibited antiviral activity of more than 67% without cytotoxicity in HeLa cells, at 100 μg/mL. Ribavirin showed anti-HRV3 activity similar to that of sakuranetin. Treatment of HRV-infected HeLa cells with sakuranetin visibly reduced CPEs.
-
Conclusion
- The inhibition of HRV production by sakuranetin is mainly due to its general antioxidant activity through inhibition of viral adsorption. Therefore, the antiviral activity of sakuranetin should be further investigated to elucidate its mode of action and prevent HRV3-mediated diseases in pathological conditions.
- Healthcare costs and loss of productivity for non-influenza viral respiratory diseases in the US have been estimated to be US dollar 39.5 billion annually [1]. Rhinoviruses (RVs) are the most common cause of viral upper respiratory diseases resulting in rhinitis, sinusitis, pharyngitis, or otitis media, and can lead to the development of bacterial superinfections [2–7]. While most individuals with an RV infection only experience mild symptoms, children, elderly individuals, immunosuppressed individuals, and those with asthma, or cystic fibrosis are predisposed to lower respiratory tract symptoms including wheezing, asthma exacerbations, and respiratory distress, which can often result in hospitalization [8,9]. Owing to numerous different serotypes (currently about 150 identified), infections are recurrent and, so far, no antiviral drug is commercially available.
- Sorbus commixta Hedl. (family Rosaceae) has been used to treat cough, asthma, and other bronchial disorders in East Asian countries, including Korea, China, and Japan [10]. It is reported to have promising antioxidant, anti-atherogenic, anti-inflammatory, anti-atherosclerotic, and vascular relaxant effects [11,12].
- Sakuranetin was first identified from the cortex of the cherry tree bark (Prunus spp.) as an aglycone of sakuranin [13]. It was recently shown to exhibit anti-inflammatory activity by inhibiting 5-lipoxygenase, antileishmanial, and antitrypanosomal activities [14,15]. In addition, sakuranetin was reported to enhance adipogenesis and insulin sensitivity of 3T3-L1 cells through upregulation of peroxisome proliferator-activated receptor γ2 (PPARγ2) [16]. Although several studies have reported the pharmacological properties of crude extracts and sakuranetin, antiviral effects of sakuranetin against human rhinoviruses (HRV) 3 have not yet been reported. This study includes the isolation of sakuranetin from the S. commixta and its antiviral activity against HRV3.
INTRODUCTION
- 1. Isolation of sakuranetin
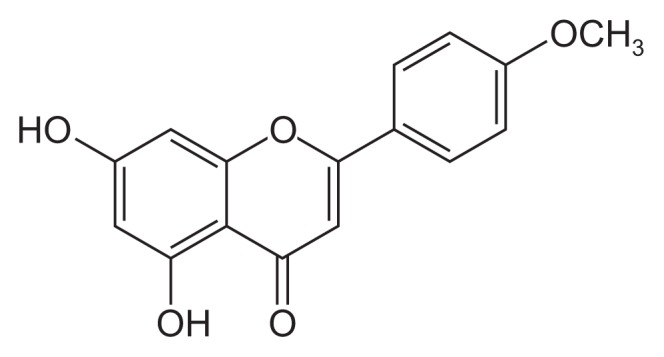
- S. commixta was obtained from Yellohip (Daejeon, Korea). The dried whole plant of S. commixta (1.2 kg) was extracted with 1 L of methanol twice at room temperature for 2 days and the extract filtered (Whatman No.2). The extract was dried by evaporation under vacuum, after which 18.84 g of solid material was obtained. The extract (18.84 g) was then suspended in distilled water and fractionated successively with n-hexane, ethylacetate, and n-butanol. The ethylacetate fraction (3 g) was subjected to silica column chromatography, and eluted with a gradient solvent system of ethylacetate: methylene chloride: methanol to obtain 18 fractions (1 Fr, 3.3 mg; 2 Fr, 30.2 mg; 3 Fr, 183.6 mg; 4 Fr, 316.0 mg; 5 Fr, 54.9 mg; 6 Fr, 54.6 mg; 7 Fr, 79.5 mg; 8 Fr, 1.7 mg; 9 Fr, 7.3 mg; 10 Fr, 76.0 mg; 11 Fr, 49.3 mg; 12 Fr, 39.8 mg; 13 Fr, 107.2 mg; 14 Fr, 515.0 mg; 15 Fr, 121.0 mg; 16 Fr, 63.2 mg; 17 Fr, 34.7 mg; 18 Fr, 35.7 mg). Fractions 1–3 contained pure sakuranetin, which was analyzed by nuclear magnetic resonance (1H and 13C) and low-resolution electron-impact mass spectrometry (Figure 1) [17].
- 2. Virus, cells, and reagents
- HRV3 was obtained from ATCC (American Type Culture Collection, Manassas, VA, USA) and propagated in human epithelioid carcinoma cervix (HeLa) cells at 32°C. Hela cells were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 0.01% antibiotic-antimycotic. Antibiotic-antimycotic, trypsin-EDTA, FBS, and MEM were obtained from Gibco BRL (Grand Island, NY, USA). The tissue culture plates were purchased from Falcon (BD Biosciences, Franklin Lakes, NJ, USA). Ribavirin and sulforhodamine B (SRB) were purchased Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of reagent grade.
- 3. Assays of antiviral activity and cytotoxicity of sakuranetin and its effect on cytopathic effects
- Antiviral activity and cytotoxicity, as well as the effect of sakuranetin on HRV3-induced cytopathic effects (CPEs) were evaluated using the SRB method using CPE reduction, as reported previously [18]. Briefly, 2 × 104 cells/well of Hela cells were seeded in MEM supplemented with 10% FBS and 0.01% antibiotic–antimycotic solution in a 96-well culture plate and incubated for 24 hours. Thereafter, medium was aspirated and cells were washed with phosphate buffered saline (PBS). Subsequently, the diluted virus suspension (0.09 mL), containing 30 mM MgCl2, 1% FBS, and 50% tissue culture infective dose (TCID50) of the virus, was added to Hela cells to produce appropriate CPEs within 48 hours after infection. Thereafter, MEM containing 50, 10, 2, and 0.4 μM of the gemcitabine (0.1 mL) were added. The culture plates were incubated at 37°C in 5% CO2 for 2 days until 50% CPE was achieved. Subsequently, the 96-well plates were washed once with PBS (100 μL). Ice-cold 70% acetone in water (100 μL) was added to each well and incubated for 30 minutes at −20°C. After eliminating the 70% acetone, plates were dried in a drying oven at 55°C for 30 minutes. The 0.4% (w/v) SRB in 1% acetic acid solution (100 μL) was added to each well and left to stand for 30 minutes at room temperature. The SRB solution was then eliminated and the plates were washed 5 times with 1% acetic acid in water before oven-drying at 55°C. Bound SRB was then solubilized with 10 mM unbuffered Tris-base (Sigma-Aldrich) solution (100 μL). After 30 minutes, the absorbance was read at 524 nm with a VERSAmax microplate reader (Molecular Devices, Palo Alto, CA, USA) with a reference absorbance determined at 650 nm. The percent protection achieved by the test compound in the HRV3-infected cells was calculated using the following equation: Antiviral activity index = [(ODt)HRV3 − (ODc)HRV3]/[(ODt)mock − (ODc)HRV3] × 100%. Ribavirin was used as the positive control and DMSO as a negative control. The morphology of HRV3-induced cells was observed using a light microscope at 32×10 magnification (AXIOVERT10; ZEISS, Oberkochen, Germany), and images were recorded.
- To evaluate cytotoxicity, Hela cells were seeded onto a 96-well culture plate at a concentration of 2 × 104 cells per well. The next day, medium was replaced with media containing serially diluted compounds. After 2 days of incubation, cytotoxicity was evaluated using the SRB assay. The culture medium was aspirated and cells were washed with PBS. The next step was performed per the antiviral activity assay described above. Results are expressed as the percentage of the controls.
MATERIALS AND METHODS
- Sakuranetin exhibited excellent antiviral activity of approximately 67% against HRV3 at 100 mg/mL and of approximately 41% at 10 mg/mL (Figure 2A). Ribavirin also exhibited good antiviral activity of approximately 60% at 100 mg/mL and weak antiviral activity of less than 11% at less than 1 mg/mL (Figure 2A).
- Cytotoxicity of each extract was assessed in parallel with antiviral activity. While sakuranetin exhibited antiviral activity, it was not toxic to Hela cells, yielding 100% cell viability at the tested concentrations (Figure 2B). Furthermore, ribavirin did not exhibit cytotoxicity in Hela cells with concentrations of 1–100 mg/mL (Figure 2B).
- The morphology of HRV3-infected cells was observed in images obtained under a light microscope. There was no difference between the mock cells (Figure 3A) or those treated with 100 mg/mL sakuranetin (Figure 3C) or ribavirin (Figure 3E) in terms of typical spread-out shapes and normal morphology at 2 days after HRV3 infection. Only infection with HRV3 without sakuranetin or ribavirin resulted in a severe CPE (Figure 3B). Treatment of HRV3-infected HeLa cells with sakuranetin reduced the formation a visible CPE (Figure 3D). Furthermore, ribavirin decreased HRV3-induced CPE (Figure 3F). Therefore, sakuranetin isolated from S. commixta exhibited inhibitory effects against HRV3 in a HeLa cell line with HRV-induced CPE reduction.
RESULTS
- Several drugs have been assessed for efficacy in treatment of HRV infections. Pleconaril is an orally absorbed viral capsid-function inhibitor that inhibits replication in 90% of RV serotypes [19]. However, the US Food and Drug Administration has not approved pleconaril because of concerns regarding the emergence of viral resistance and the reduced effectiveness of oral contraceptives among women using pleconaril [20]. Hence, the lack of effective therapy for HRV infections necessitates studies on new antiviral agents.
- Many viruses can induce cell death, leading to lysis of infected cells [21]. In the late stages of HRV3 infection, morphological changes commonly known as CPE, can be observed microscopically. The morphology of HeLa cells after HRV3 infection was significantly different from that after treatment with sakuranetin.
- Flavonoids constitute a large class of polyphenolic compounds and are integral components that are abundant in our daily diet, in vegetables, fruits, and plant-derived beverages. Numerous studies have suggested that flavonoids may protect against carcinogens coronary heart disease, bone loss, and many other age-related diseases [22]. Several previous reports have documented that flavonoids possess anti-human immunodeficiency virus (HIV) [23]. Anti-hepatitis B virus activity and antiviral activities of flavonoids have also been observed against several other viruses [24]. Sakuranetin is a flavonoid phytoalexin that serves as a plant antibiotic and exists in Prunus and several other plant species [25]. In this study, the anti-HRV3 activity of sakuranetin was evaluated in vitro. Sakuranetin exhibited anti-HRV3 activity in the CPE reduction assay.
- During the past few years, efforts have been made to increase the number of antiviral agents and a few belong to the class of nucleoside analogs such as ribavirin [26]. A previous study reported that ribavirin inhibited Lassa virus replication [27]. Ribavirin also inhibits Zika virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice [28]. Although ribavirin has a high efficacy as an antiviral agent, certain viruses that acquired resistance to ribavirin have been isolated from various virus populations and detected in some patients [29]. In the present study, ribavirin showed antiviral activity in HRV3-infected HeLa cells.
- In conclusion, sakuranetin was shown to be effective against HRV3. Further studies are required to understand its antiviral mechanism to develop a novel drug for treating HRV3 infections.
DISCUSSION
-
Acknowledgements
- This study was supported (in part) by Research Funds of Kwangju Women’s University in 2017 (KWUI17-033).
ACKNOWLEDGMENTS
-
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
Article information
- 1. Fendrick AM, Monto AS, Nightengale B, et al. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med 2003;163:487−94. https://doi.org/10.1001/archinte.163.4.487. PMID: 10.1001/archinte.163.4.487. PMID: 12588210.ArticlePubMed
- 2. Jakab GJ. Mechanisms of bacterial superinfections in viral pneumonias. Schweiz Med Wochenschr 1985;115:75−86. PMID: 3883482.PubMed
- 3. Monto AS, Bryan ER, Ohmit S. Rhinovirus infections in Tecumseh, Michigan: frequency of illness and number of serotypes. J Infect Dis 1987;156:43−9. https://doi.org/10.1093/infdis/156.1.43. PMID: 10.1093/infdis/156.1.43. PMID: 3036962.ArticlePubMed
- 4. Hament JM, Kimpen JL, Fleer A, et al. Respiratory viral infection predisposing for bacterial disease: a concise review. FEMS Immunol Med Microbiol 1999;26:189−95. https://doi.org/10.1111/j.1574-695X.1999.tb01389.x. PMID: 10.1111/j.1574-695X.1999.tb01389.x. PMID: 10575129.ArticlePubMed
- 5. Ishizuka S, Yamaya M, Suzuki T, et al. Effects of rhinovirus infection on the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells. J Infect Dis 2003;188:1928−39. https://doi.org/10.1086/379833. PMID: 10.1086/379833. PMID: 14673774.ArticlePubMed
- 6. Monto AS. Epidemiology of viral respiratory infections. Am J Med 2002;112(Suppl 6A). 4S−12S. https://doi.org/10.1016/S0002-9343(01)01058-0. PMID: 10.1016/S0002-9343(01)01058-0. PMID: 11955454.ArticlePubMed
- 7. Bosch AA, Biesbroek G, Trzcinski K, et al. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog 2013;9:e1003057https://doi.org/10.1371/journal.ppat.1003057. PMID: 10.1371/journal.ppat.1003057. PMID: 23326226.ArticlePubMedPMC
- 8. Johnston SL, Pattemore PK, Sanderson G, et al. Community study of role of viral infections in exacerbations of asthma in 9–11 year old children. BMJ 1995;310:1225−9. https://doi.org/10.1136/bmj.310.6989.1225. PMID: 10.1136/bmj.310.6989.1225. PMID: 7767192.ArticlePubMedPMC
- 9. Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. J Allergy Clin Immunol 2005;116:267−73. https://doi.org/10.1016/j.jaci.2005.06.003. PMID: 10.1016/j.jaci.2005.06.003. PMID: 16083778.ArticlePubMed
- 10. Kang DG, Sohn EJ, Lee AS, et al. Methanol extract of Sorbus commixta cortex prevents vascular inflammation in rats with a high fructose-induced metabolic syndrome. Am J Chin Med 2007;35:265−77. https://doi.org/10.1142/S0192415X07004801. PMID: 10.1142/S0192415X07004801. PMID: 17436367.ArticlePubMed
- 11. Bae JT, Sim GS, Kim JH, et al. Antioxidative activity of the hydrolytic enzyme treated Sorbus commixta Hedl. and its inhibitory effect on matrix metalloproteinase-1 in UV irradiated human dermal fibroblasts. Arch Pharm Res 2007;30:1116−23. PMID: 10.1007/BF02980246. PMID: 17958329.ArticlePubMed
- 12. Sohn EJ, Kang DG, Mun YJ, et al. Anti-atherogenic effects of the methanol extract of Sorbus cortex in atherogenic-diet rats. Biol Pharm Bull 2005;28:1444−9. https://doi.org/10.1248/bpb.28.1444. PMID: 10.1248/bpb.28.1444. PMID: 16079490.ArticlePubMed
- 13. Asahina Y. Ueber das sakuranin, ein neues glykosid der rinde von Prunus pseudo-Cerasus lindl. var. sieboldi Maxim. Arch Pharm 1908;246:259−72. In German. https://doi.org/10.1002/ardp.19082460404. PMID: 10.1002/ardp.19082460404.Article
- 14. Zhang X, Hung TM, Phuong PT, et al. Anti-inflammatory activity of flavonoids from Populus davidiana. Arch Pharm Res 2006;29:1102−8. PMID: 10.1007/BF02969299. PMID: 17225458.ArticlePubMed
- 15. Miyazawa M, Kinoshita H, Okuno Y. Antimutagenic activity of Sakuranetin from Prunus jamasakura. J Food Sci 2003;68:52−6. https://doi.org/10.1111/j.1365-2621.2003.tb14113.x. PMID: 10.1111/j.1365-2621.2003.tb14113.x.Article
- 16. Saito T, Abe D, Sekiya K. Sakuranetin induces adipogenesis of 3T3-L1 cells through enhanced expression of PPARgamma2. Biochem Biophys Res Commun 2008;372:835−9. https://doi.org/10.1016/j.bbrc.2008.05.146. PMID: 10.1016/j.bbrc.2008.05.146. PMID: 18522800.ArticlePubMed
- 17. Taguchi L, Pinheiro NM, Olivo CR, et al. A flavanone from Baccharis retusa (Asteraceae) prevents elastase-induced emphysema in mice by regulating NF-κB, oxidative stress and metalloproteinases. Respir Res 2015;16:79https://doi.org/10.1186/s12931-015-0233-3. PMID: 10.1186/s12931-015-0233-3. PMID: 26122092.ArticlePubMedPMC
- 18. Choi HJ, Kim JH, Lee CH, et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res 2009;81:77−81. https://doi.org/10.1016/j.antiviral.2008.10.002. PMID: 10.1016/j.antiviral.2008.10.002. PMID: 18992773.ArticlePubMed
- 19. Pevear DC, Hayden FG, Demenczuk TM, et al. Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses. Antimicrob Agents Chemother 2005;49:4492−9. https://doi.org/10.1128/AAC.49.11.4492-4499.2005. PMID: 10.1128/AAC.49.11.4492-4499.2005. PMID: 16251287.ArticlePubMedPMC
- 20. Fleischer R, Laessig K. Safety and efficacy evaluation of pleconaril for treatment of the common cold. Clin Infect Dis 2003;37:1722https://doi.org/10.1086/379830. PMID: 10.1086/379830. PMID: 14689362.Article
- 21. Song JH, Shim JK, Choi HJ. Quercetin 7-rhamnoside reduces porcine epidemic diarrhea virus replication via independent pathway of viral induced reactive oxygen species. Virol J 2011;8:460https://doi.org/10.1186/1743-422X-8-460. PMID: 10.1186/1743-422X-8-460. PMID: 21967756.ArticlePubMedPMC
- 22. Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther 2002;96:67−202. https://doi.org/10.1016/S0163-7258(02)00298-X. PMID: 10.1016/S0163-7258(02)00298-X. PMID: 12453566.ArticlePubMed
- 23. Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol 2003;95:412−27. https://doi.org/10.1046/j.1365-2672.2003.02026.x. PMID: 10.1046/j.1365-2672.2003.02026.x. PMID: 12911688.ArticlePubMed
- 24. Li J, Huang H, Zhou W, et al. Anti-hepatitis B virus activities of Geranium carolinianum L. extracts and identification of the active components. Biol Pharm Bull 2008;31:743−7. https://doi.org/10.1248/bpb.31.743. PMID: 10.1248/bpb.31.743. PMID: 18379075.ArticlePubMed
- 25. Kim KY, Kang H. Sakuranetin inhibits inflammatory enzyme, cytokine, and costimulatory molecule expression in macrophages through modulation of JNK, p38, and STAT1. Evid Based Complement Alternat Med 2016;2016:9824203https://doi.org/10.1155/2016/9824203. PMID: 10.1155/2016/9824203. PMID: 27668006.ArticlePubMedPMC
- 26. De Clercq E. Antiviral drugs in current clinical use. J Clin Virol 2004;30:115−33. https://doi.org/10.1016/j.jcv.2004.02.009. PMID: 10.1016/j.jcv.2004.02.009. PMID: 15125867.ArticlePubMed
- 27. Carrillo-Bustamante P, Nguyen THT, Oestereich L, et al. Determining ribavirin’s mechanism of action against Lassa virus infection. Sci Rep 2017;7:11693https://doi.org/10.1038/s41598-017-10198-0. PMID: 10.1038/s41598-017-10198-0. PMID: 28916737.ArticlePubMedPMC
- 28. Kamiyama N, Soma R, Hidano S, et al. Ribavirin inhibits Zika virus (ZIKV) replication in vitro and suppresses viremia in ZIKV-infected STAT1-deficient mice. Antiviral Res 2017;146:1−11. https://doi.org/10.1016/j.antiviral.2017.08.007. PMID: 10.1016/j.antiviral.2017.08.007. PMID: 28818572.ArticlePubMedPMC
- 29. Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol 2006;16:37−48. https://doi.org/10.1002/rmv.483. PMID: 10.1002/rmv.483. PMID: 16287208.ArticlePubMed
REFERENCES
Figure 2Antiviral activity of sakuranetin against human rhinovirus (HRV) 3 in human epithelioid carcinoma cervix (HeLa) cells. Antiviral activity of the three extracts against HRV3 in HeLa cells is shown; (A) Antiviral activity of sakuranetin against HRV3 in HeLa cells. (B) Cytotoxicity of sakuranetin in HeLa cells. The diluted virus suspension, containing 30 mM MgCl2, 1% fetal bovine serum, and 50% tissue culture infective dose of the virus, was added to HeLa cells to produce the appropriate cytopathic effect (CPE) within 48 hours after infection. The antiviral activity and cytotoxicity of sakuranetin were investigated through the sulforhodamine B (SRB) assay with CPE reduction. Results are presented as the mean percentage values obtained from three independent experiments performed in triplicate ± standard deviation.


Figure 3The effect of sakuranetin on human rhinovirus (HRV) 3-induced cytopathic effect (CPE). The effects of sakuranetin on HRV3-induced CPE are shown. Culture medium in 96-well tissue culture plates was aspirated and the cells were washed with phosphate buffered saline. Thereafter, 0.09 mL of the diluted virus suspension, containing 30 mM MgCl2, 1% fetal bovine serum, and 50% tissue culture infective dose of the virus and 0.01 mL of medium were added to human epithelioid carcinoma cervix (HeLa) cells to produce the appropriate CPE within 48 hours after infection, and then sakuranetin or ribavirin (100 mg/mL) was added. After incubation at 32°C and 5% CO2 for 2 days, the cells stained by SRB, and cellular morphology was studied using photographs taken under a light microscope (×400). (A) Non-infected cells; (B) HRV3-infected cells without sakuranetin or ribavirin treatment; (C) non-infected cells treated with sakuranetin; (D) virus-infected cells treated with sakuranetin; (E) non-infected cells with treated ribavirin; (F) virus-infected cells treated with ribavirin.
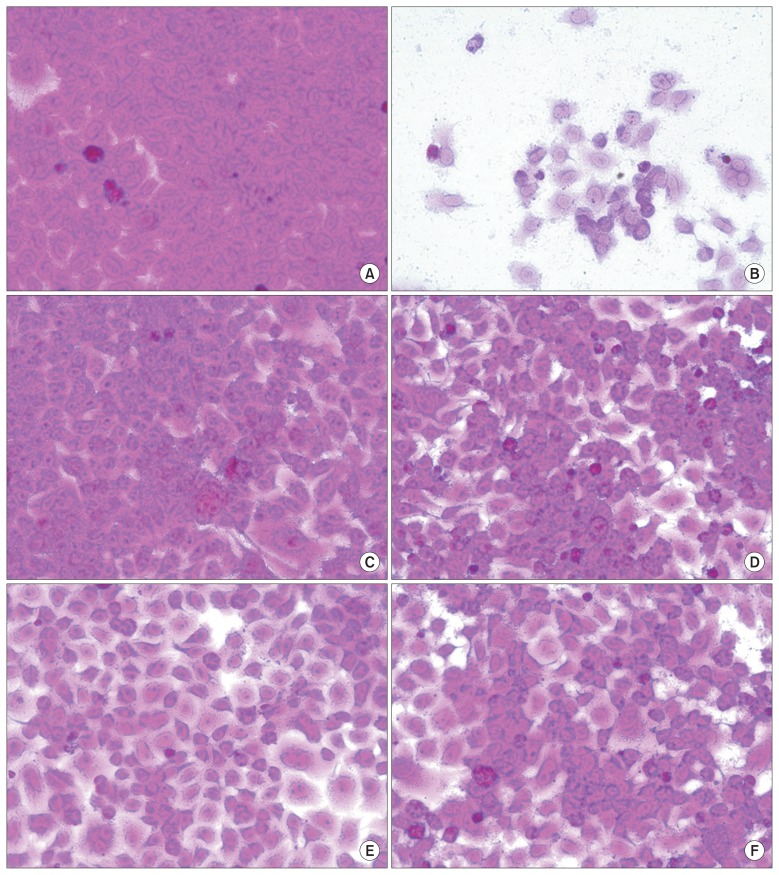

Figure & Data
References
Citations
Citations to this article as recorded by 

- Interaction of sakuranetin with unsaturated lipids forming Langmuir monolayers at the air-water interface: A biomembrane model
Matheus Lima de Souza, André Campos Machado, Henrique Barbosa, João Henrique Ghilardi Lago, Luciano Caseli
Colloids and Surfaces B: Biointerfaces.2024; 234: 113747. CrossRef - Biofortified Rice Provides Rich Sakuranetin in Endosperm
Yao Zhao, Jitao Hu, Zhongjing Zhou, Linying Li, Xueying Zhang, Yuqing He, Chi Zhang, Junmin Wang, Gaojie Hong
Rice.2024;[Epub] CrossRef - Sakuranetin and its therapeutic potentials – a comprehensive review
Md. Junaid, Bristy Basak, Yeasmin Akter, Syeda Samira Afrose, Afsana Nahrin, Rashiduzzaman Emran, Md. Shahinozzaman, Shinkichi Tawata
Zeitschrift für Naturforschung C.2023; 78(1-2): 27. CrossRef - Comparative metabolomics of flavonoids in twenty vegetables reveal their nutritional diversity and potential health benefits
Han Tao, Yao Zhao, Linying Li, Yuqing He, Xueying Zhang, Ying Zhu, Gaojie Hong
Food Research International.2023; 164: 112384. CrossRef - Rice Phytoalexins: Half a Century of Amazing Discoveries; Part I: Distribution, Biosynthesis, Chemical Synthesis, and Biological Activities
Alessio Valletta, Lorenzo Maria Iozia, Laura Fattorini, Francesca Leonelli
Plants.2023; 12(2): 260. CrossRef - Ethanolamine phospholipids at the air-water interface as cell membranes models of microorganisms to study the nanotoxicology of sakuranetin
Guilherme Henrique da Cruz Ramos Pires, Henrique Barbosa, Roberto Baptista Pereira Almeida, João Henrique Ghilardi Lago, Luciano Caseli
Thin Solid Films.2023; 770: 139768. CrossRef - Antiviral Activity of Flavonoids Against Non-polio Enteroviruses
Hwa-Jung Choi
Journal of Bacteriology and Virology.2023; 53(1): 29. CrossRef - Novel anti‑hepatitis B virus flavonoids sakuranetin and velutin fromRhus retinorrhoea
Sarfaraz Ahmed, Mohammad Parvez, Mohammed Al‑Dosari, Mazin Abdelwahid, Tawfeq Alhowiriny, Adnan Al‑Rehaily
Molecular Medicine Reports.2023;[Epub] CrossRef - Antiviral Activity of Flavonoids from Geopropolis of the Brazilian Jandaira Bee against Zika and Dengue Viruses
Poliana Gomes da Silva, Elton José Ferreira Chaves, Tania Maria Sarmento Silva, Gerd Bruno Rocha, Willyenne Marília Dantas, Ronaldo Nascimento de Oliveira, Lindomar José Pena
Pharmaceutics.2023; 15(10): 2494. CrossRef - Metabolic engineering in Streptomyces albidoflavus for the biosynthesis of the methylated flavonoids sakuranetin, acacetin, and genkwanin
Álvaro Pérez-Valero, Suhui Ye, Patricia Magadán-Corpas, Claudio J. Villar, Felipe Lombó
Microbial Cell Factories.2023;[Epub] CrossRef - Antiviral Activity of Quercetin-3-Glucoside Against Non-Polio Enterovirus
Hwa-Jung Choi
Journal of Bacteriology and Virology.2022; 52(1): 20. CrossRef - Sakuranetin interacting with cell membranes models: Surface chemistry combined with molecular simulation
Guilherme Henrique da Cruz Ramos Pires, Vitor Torres Freire, Rafael Guimarães Pereira, Leonardo José Amaral de Siqueira, Eric Umehara, João Henrique Ghilardi Lago, Luciano Caseli
Colloids and Surfaces B: Biointerfaces.2022; 216: 112546. CrossRef - Production of (2S)-sakuranetin from (2S)-naringenin in Escherichia coli by strengthening methylation process and cell resistance
Qiumeng Sun, Song Gao, Shiqin Yu, Pu Zheng, Jingwen Zhou
Synthetic and Systems Biotechnology.2022; 7(4): 1117. CrossRef - Sakuranetin State of the Art: Physical Properties, Biological Effects, and Biotechnological Trends
Leonardo Ribeiro Bernardo, Anna Rafaela Cavalcante Braga
Industrial Biotechnology.2022; 18(6): 341. CrossRef - Stevia Genus: Phytochemistry and Biological Activities Update
Jimena Borgo, Laura C. Laurella, Florencia Martini, Cesar A. N. Catalán, Valeria P. Sülsen
Molecules.2021; 26(9): 2733. CrossRef - Phytochemistry and teratogenic potential of Mimosa tenuiflora (willd.) poir. (Fabaceae) in ruminants: A systematic review
José Jailson Lima Bezerra, Anderson Angel Vieira Pinheiro, Ricardo Barbosa Lucena
Toxicon.2021; 195: 78. CrossRef - DO WE KNOW RHINOVIRUSES AND THEIR CLINICAL IMPACT?
Irina Georgieva, Asya Stoyanova, Svetla Angelova, Savina Stoitsova, Silvia Voleva, Neli Korsun, Lubomira Nikolaeva-Glomb
PROBLEMS of Infectious and Parasitic Diseases.2021; 49(1): 5. CrossRef - Flavonoids as Antiviral Agents for Enterovirus A71 (EV-A71)
Salima Lalani, Chit Laa Poh
Viruses.2020; 12(2): 184. CrossRef - A Review on Sources and Pharmacological Aspects of Sakuranetin
Monika Stompor
Nutrients.2020; 12(2): 513. CrossRef - Ethnopharmacologically important but underestimated genus Sorbus: a comprehensive review
Agnieszka Sołtys, Agnieszka Galanty, Irma Podolak
Phytochemistry Reviews.2020; 19(2): 491. CrossRef - The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential
Viive Sarv, Petras Rimantas Venskutonis, Rajeev Bhat
Antioxidants.2020; 9(9): 813. CrossRef - Suppression of influenza B virus replication by sakuranetin and mode of its action
Dur‐Han Kwon, Jeong‐Hun Ji, Soon‐Ho Yim, Byoung‐Soo Kim, Hwa‐Jung Choi
Phytotherapy Research.2018; 32(12): 2475. CrossRef


 PubReader
PubReader Cite
Cite
