Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 9(6); 2018 > Article
-
Original Article
Chemical Constituents of Essential Oils Possessing Anti-Influenza A/WS/33 Virus Activity - Hwa-Jung Choi
-
Osong Public Health and Research Perspectives 2018;9(6):348-353.
DOI: https://doi.org/10.24171/j.phrp.2018.9.6.09
Published online: December 31, 2018
Department of Beauty Science, Kwangju Women’s University, Gwangju, Korea
- *Corresponding author: Hwa-Jung Choi, Department of Beauty Science, Kwangju Women’s University, Gwangju, South Korea, E-mail: rerived@kwu.ac.kr
• Received: April 30, 2018 • Revised: November 23, 2018 • Accepted: November 27, 2018
Copyright ©2018, Korea Centers for Disease Control and Prevention
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- This study was conducted to determine whether essential oils had anti-influenza A/WS/33 virus activity and whether there were specific compounds associated with this activity.
-
Methods
- There were 63 essential oils evaluated for anti-influenza (A/WS/33 virus) activity using a cytopathic effect reduction method. The chemical composition of the anti-influenza essential oils was phytochemically analyzed by gas chromatography-mass spectrometry.
-
Results
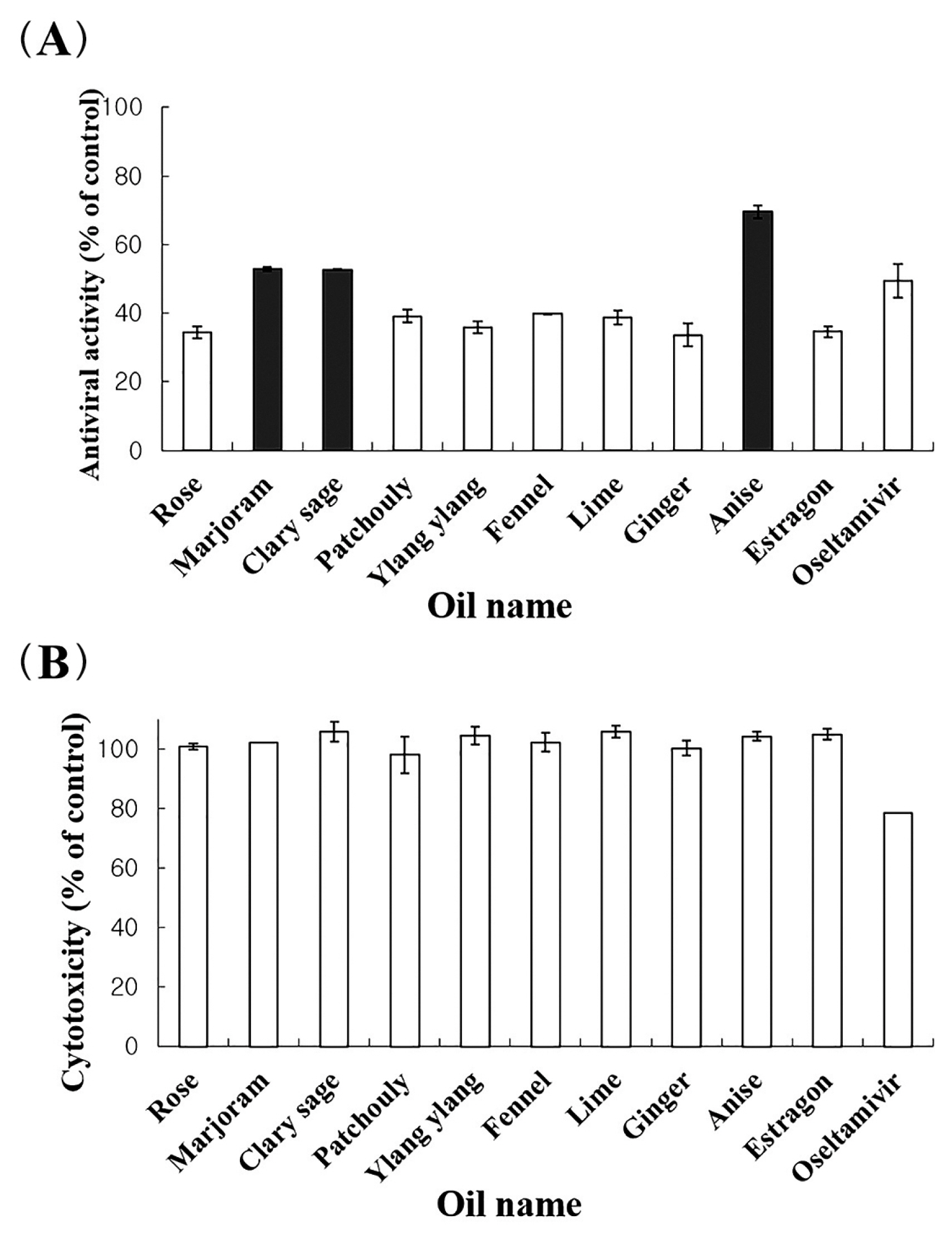
- The antiviral assays demonstrated that 11 of the 62 essential oils (100 μg/mL) possessed anti-influenza activity, reducing visible cytopathic effects of influenza A/WS/33 virus activity by > 30%. Furthermore, marjoram, clary sage and anise oils exhibited anti-influenza A/WS/33 virus activity of > 52.8%. However, oseltamivir (the anti-influenza A and B drug), showed cytotoxicity at the same concentration (100 μg/mL) as the essential oils. The chemical composition detected by GC–MS analysis, differed amongst the 3 most potent anti-viral essential oils (marjoram, clary sage and anise oils) except for linalool, which was detected in all 3 essential oils.
-
Conclusion
- This study demonstrated anti-influenza activity in 11 essential oils tested, with marjoram, clary sage and anise essential oils being the most effective at reducing visible cytopathic effects of the A/WS/33 virus. All 3 oils contained linalool, suggesting that this may have anti-influenza activity. Further investigation is needed to characterize the antiviral activity of linalool against influenza A/WS/33 virus.
- Influenza viruses are enveloped RNA viruses that infect humans and animals, and cause respiratory complications resulting in high morbidity rates [1]. The preferred treatment for influenza infection are neuraminidase inhibitors (oseltamivir and zanamivir) [2]. However, their use has been limited by side-effects, and the emergence of resistant viral strains [3,4].
- Essential oils are known to possess multifunctional properties other than their traditional roles, as various biological agents have been shown to demonstrate anti-bacterial, anti-fungal, and anti-inflammatory activities [5,6]. Several studies have documented antiviral activity of essential oils [7–9]. Recent studies have demonstrated that eucalyptus essential oil showed inhibitory effects on adenovirus and mumps virus [10]. A previous study showed that the volatile oil from Cynanchum stauntonii possessed direct inhibitory activity against influenza virus [11].
- In this present study, the potential anti-viral properties of 62 essential oils on influenza A/WS/33 virus have been analyzed using the cytopathic effect (CPE) reduction method. Marjoram (Thymus mastichina L.), clary sage (Salvia sclarea L.) and anise (Pimpinella anisum L.) oils showed anti-influenza A/WS/33 activity and were phytochemically examined by gas chromatography-mass spectrometry (GC-MS) analysis, and their chemical compositions analyzed.
Introduction
- 1. Materials and cell culture
- Essential plant oils (n = 62) were purchased from UNIQ F&F Co., Ltd. (Seoul, Korea) and listed in Table 1. The samples were deposited in Seoul National University herbarium. To test the materials, the oils were solubilized in dimethylsulfoxide to give a final concentration of 10 mg/mL, and stored at -20°C until further use.
- The Influenza A/WS/33 virus was provided by ATCC (American Type Culture Collection, Manassas, VA, USA) and propagated in Madin-Darby canine kidney (MDCK) cells at 37°C. MDCK cells were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum and 0.01% antibiotic-antimycotic solution. Antibiotic-antimycotic solution, trypsin- ethylenediamine tetra-acetic acid (EDTA), fetal bovine serum and MEM were supplied by Gibco BRL (Grand Island, NY). Tissue culture plates were purchased from Falcon (BD Biosciences, Franklin Lakes, NJ). Sulforhodamine B (SRB) was purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were of reagent grade. Oseltamivir (F. Hofmann-La Roche Ltd, Switzerland) was purchased from a pharmacy in Korea as prescribed by a traditional Korean doctor.
- 2. Antiviral and cytotoxicity assay
- Anti-influenza (A/WS/33 virus) activity of essential oils was determined by the CPE reduction method [12]. There were 2 × 104 MDCK cells/well seeded into a 96-well culture plate in MEM supplemented with trypsin-EDTA containing 0.01% antibiotic–antimycotic solution and incubated at 37°C in 5% CO2 for 24 hours. Thereafter, the medium was aspirated and cells were washed with phosphate buffered saline (PBS). Subsequently, the diluted virus suspension (0.09 mL) containing 50% tissue culture infective dose (TCID50) of the virus, was added to MDCK cells to produce an appropriate CPE within 48 hours after infection. Thereafter, MEM containing essential oils in 100 μg/mL were added to each well. The culture plates were incubated at 37°C in 5% CO2 for 2 days until 50% CPE was achieved. Subsequently, the 96-well plates were washed once with PBS (100 mL). Ice-cold 70% acetone in water (100 mL) was added to each well and incubated for 30 minutes at −20°C. After 70% acetone had evaporated, the plates were dried in an oven at 55°C for 30 minutes. The plate was developed using 0.4% (w/v) SRB in 1% acetic acid solution (100 mL) which was added to each well and left to stand for 30 minutes at room temperature. The excess SRB solution was removed by washing the plates 5 times with 1% acetic acid in water, then the plate was dried at 55°C. Bound SRB was then solubilized with 10 mM unbuffered Tris-base (SigmaAldrich) solution (100 mL). After 30 minutes, the absorbance was read at 524 nm with a VERSAmax microplate reader (Molecular Devices, Palo Alto, CA, USA) with a reference absorbance determined at 650 nm. The percent protection achieved by the test compound in the influenza A/WS/33 virus-infected cells was calculated using the equation below. Oseltamivir was used as the positive control and dimethylsulfoxide as a negative control. The morphology of influenza A/WS/33 virus-infected cells was observed using a light microscope at 32 × 10 magnification (AXIOVERT10; ZEISS, Oberkochen, Germany), and images were recorded.
- To evaluate cytotoxicity, MDCK cells were seeded onto a 96-well culture plate at a concentration of 2 × 104 cells per well. The next day, the medium was replaced with medium containing serially diluted compounds. After 2 days of incubation at 37°C in 5% CO2, cytotoxicity was evaluated using the SRB assay. The culture medium was aspirated and cells were washed with PBS. The next step was performed per the antiviral activity assay described above. Results were expressed as the percentage of the controls.
- 3. Effect of essential oils on morphological changes of influenza virus-induced MDCK cells
- The effect of essential oils on influenza virus-induced CPE was observed. Briefly, MDCK cells were seeded onto a 96-well culture plate at a concentration of 2 × 104 cells per well. The next day, the culture medium was removed and the cells were washed with PBS. Diluted virus suspension (0.09 mL) and 0.01 mL of medium supplemented with trypsin-EDTA containing essential oils at 100 μg/mL were added to each well. After incubation at 37°C in 5% CO2 for 2 days, the morphology of cells was observed under the microscope at 32 × 10 magnifications (AXIOVERT10, ZEISS, Germany), and images were recorded.
- 4. Gas chromatography
- Gas chromatography analysis was performed on an Agilent 6890N equipped with a DB-1MS column (30 mm × 0.25 mm i.d., 0.25 μm film thickness, J&W Scientific, Folsom, CA). The oven temperature was programmed as: isothermal at 40°C for 1 minute, then raised to 250°C (6°C/minute) and held at this temperature for 4 minutes. Helium was used as the carrier gas at the rate of 1.5 mL/minute in split mode (50:1 ratio). The constituents of the plant essential oil were identified by comparing their GC retention indices (RI). The RI of the constituents for each plant essential oil were identified by co-injection of essential oil and a mixture of aliphatic hydrocarbons (C8–C20; Sigma-Aldrich, St. Louis, USA). RI was calculated using the equation proposed by van Den Dool and Kratz (1963) [13].
- 5. Gas chromatography-mass spectrometry
- The oils (marjoram, clary sage and anise) were analyzed further on a gas chromatograph (Agilent 6890N)-mass spectrometer (Agilent 5973N MSD) equipped with a DB-5MS column (30 m × 0.25 mm i.d., 0.25 μm film thickness, J & W Scientific, Folsom, CA). The oven temperature was programmed as described previously. Helium was used as the carrier gas at the rate of 1.0 mL/minute. The effluent of the GC column was introduced directly into the source of the MS via a transfer line (250°C). Ionization voltage was 70 eV and the ion source temperature was 230°C. Scan range was 41–450 amu. Compounds were identified by comparison of mass spectra of each peak with those of authentic samples in the NIST MS library.
Materials and Methods
- 1. Marjoram, clary sage and anise oils possess anti-influenza A/WS/33 virus activity
- Anti-influenza (A/WS/33 virus) activity of all essential oils were investigated. Eleven essential oils amongst the 62 essential oils tested possessed antiviral activity of > 30% and did not show cytotoxicity at a concentration of 100 μg/mL (Figure 1A). There were 3 oils (marjoram, clary sage and anise oils) that exhibited a higher anti-influenza activity (> 52%) than oseltamivir, with no cytotoxicity at a concentration of 100 μg/mL. However, oseltamivir showed cytotoxicity at this concentration (Figure 1B).
- 2. Marjoram, clary sage and anise oils reduce anti-influenza A/WS/33 virus-induced morphological changes
- The effects of essential oils on influenza A/WS/33 virus-induced CPE were investigated (Figure 2). After a 2-day infection of MDCK cells with influenza A/WS/33 virus, mock cells (Figure 2A) or cells treated with 100 μg/mL essential oils (Figures 2E, 2G and 2I) showed typical morphology. At this concentration (100 μg/mL), there were no signs of cytotoxicity induced by the essential oils. However, oseltamivir was weakly toxic to MDCK cells at concentration of 100 μg/mL (Figure 2C). Infection with influenza A/WS/33 virus, in the absence of essential oils, resulted in a severe CPE (Figure 2B). Addition of the essential oils to the influenza infected MDCK cells, inhibited the formation of a visible CPE (Figures 2F, 2H and 2J). However, the addition of oseltamivir to influenza A/WS/33 virus-infected MDCK cell resulted in a small reduction in CPE (Figure 2D). Thus, the CPE of the virus infection was shown to be prevented by the presence of the essential oils.
- 3. Chemical compositions of the marjoram, clary sage and anise oils
- The chemical compositions of marjoram, clary sage and anise essential oils are shown in Tables 2 to 4. A total of 10 compounds were identified in marjoram oil by GC and GC-MS analysis (Table 2). Among the identified compounds, 1,8-cineole (64.61%) was the most abundant compound followed by linalool (15.28%) and β–pinene (5.81%; Table 2). The chemical compositions of clary sage oil showed linalayl acetate (61.16%) to be the highest concentration, followed by linalool (22.06%) and α–terpineol (4.21%; Table 3). The chemical compositions of anise oil were trans-anethole (82.78%), estragole (8.21%) and linalool (2.74%; Table 4).
Results
- Many antiviral compounds have been developed against the influenza virus, the long-term efficacy of which is often limited due to toxicity or the emergence of drug-resistant virus mutants [14]. Hence, new approaches for the control of highly pathogenic influenza viruses must be explored.
- Previous studies showed antiviral activity of essential oils against DNA viruses such as herpes simplex virus [7,8]. Ocimum basilicum (OB), also known as sweet basil, showed antiviral activities against DNA viruses (herpes viruses (HSV), adenoviruses (ADV) and hepatitis B virus and RNA viruses (coxsackievirus B1 (CVB1) and enterovirus 71 (EV71). Apigenin, linalool and ursolic acid isolated from crude aqueous and ethanolic extracts of OB, exhibited a broad spectrum of antiviral activity against these viruses [15].
- In this work, marjoram, clary sage and anise oils exhibited strong anti-influenza A/WS/33 virus activity. Linalool was a common constituent in the chemical compositions of marjoram, clary sage and anise oils. Therefore, the anti-influenza A/WS/33 activity of marjoram, clary sage and anise oils appeared to be associated with linalool. However, further studies will be required to explore the anti-influenza A/WS/33 virus effects of linalool.
- In conclusion, marjoram, clary sage and anise oils showed interesting anti-influenza A/WS/33 activity. A common constituent of the 3 oils was linalool. Therefore, further studies are required to understand whether or not linalool possesses antiviral activity against influenza A/WS/33 virus.
Discussion
-
Acknowledgements
- This paper was supported (in part) by Research Funds of Kwangju Women’s University in 2018 (KWUI18-035).
Acknowledgments
-
Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Article information
- 1. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev 1992;56(1). 152−79. PMID: 1579108. PMID: 372859.ArticlePubMedPMC
- 2. Garman E, Laver G. Controlling influenza by inhibiting the virus’s neuraminidase. Curr Drug Targets 2004;5(2). 119−36. PMID: 10.2174/1389450043490604. PMID: 15011946.ArticlePubMed
- 3. Kiso M, Mitamura K, Sakai-Tagawa Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 2004;364(9436). 759−65. PMID: 10.1016/S0140-6736(04)16934-1. PMID: 15337401.ArticlePubMed
- 4. Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005;437(7062). 1108PMID: 10.1038/4371108a. PMID: 16228009.ArticlePubMedPDF
- 5. Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol 1999;86(6). 985−90. PMID: 10.1046/j.1365-2672.1999.00780.x. PMID: 10438227.ArticlePubMed
- 6. Meepagala KM, Sturtz G, Wedge DE. Antifungal constituents of the essential oil fraction of Artemisia dracunculus L. var. dracunculus. J Agric Food Chem 2002;50(24). 6989−92. PMID: 10.1021/jf020466w. PMID: 12428948.ArticlePubMed
- 7. Tragoolpua Y, Jatisatienr A. Anti-herpes Simplex Virus Activities of Eugenia caryophyllus (Spreng). Bullock & S. G. Harrison and Essential Oil, Eugenol Phytother Res 2007;21(12). 1153−8. PMID: 17628885.
- 8. Koch C, Reichling J, Schneele J, et al. Inhibitory effect of essential oils against herpes simplex virus type 2. Phytomed 2008;15(1–2). 71−8. PMID: 10.1016/j.phymed.2007.09.003.Article
- 9. Schnitzler P, Schuhmachera A, Astania A, et al. Melissa officinalis oil affects infectivity of enveloped herpesviruses. Phytomed 2008;15(9). 734−40. PMID: 10.1016/j.phymed.2008.04.018.Article
- 10. Cermelli C, Fabio A, Fabio G, et al. Effect of eucalyptus essential oil on respiratory bacteria and viruses. Curr Microbiol 2008;56(1). 89−92. PMID: 10.1007/s00284-007-9045-0.ArticlePubMedPDF
- 11. Yang ZC, Wang BC, Yang XS, et al. Chemical composition of the volatile oil from Cynanchum stauntonii and its activities of anti-influenza virus. Colloids and Surfaces B: Biointerfaces 2005;43(3–4). 198−202. PMID: 10.1016/j.colsurfb.2005.05.003.ArticlePubMed
- 12. Choi HJ, Kim JH, Lee CH, et al. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res 2009;81(1). 77−81. PMID: 10.1016/j.antiviral.2008.10.002.ArticlePubMed
- 13. van Den Dool H, Kratz PD. Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr 1963;11:463−71. PMID: 10.1016/S0021-9673(01)80947-X.ArticlePubMed
- 14. Hayden FG. Antivirals for influenza: historical perspectives and lessons learned. Antivir Res 2006;71(1–2). 372−8. PMID: 10.1016/j.antiviral.2006.05.016. PMID: 16815563.ArticlePubMed
- 15. Chiang LC, Ng LT, Cheng PW, et al. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol 2005;32(10). 811−6. PMID: 10.1111/j.1440-1681.2005.04270.x. PMID: 16173941.ArticlePubMed
References
Figure 1
Antiviral activity of essential oils on influenza A/WS/33 virus.
Virus suspension and media containing essential oil (100 μg/mL) were added to the cells. After incubation 2 days, antiviral activity was investigated using the CPE reduction assay.
Results are presented as the mean percentage values obtained from 3 independent experiments carried out in triplicate ± SD.
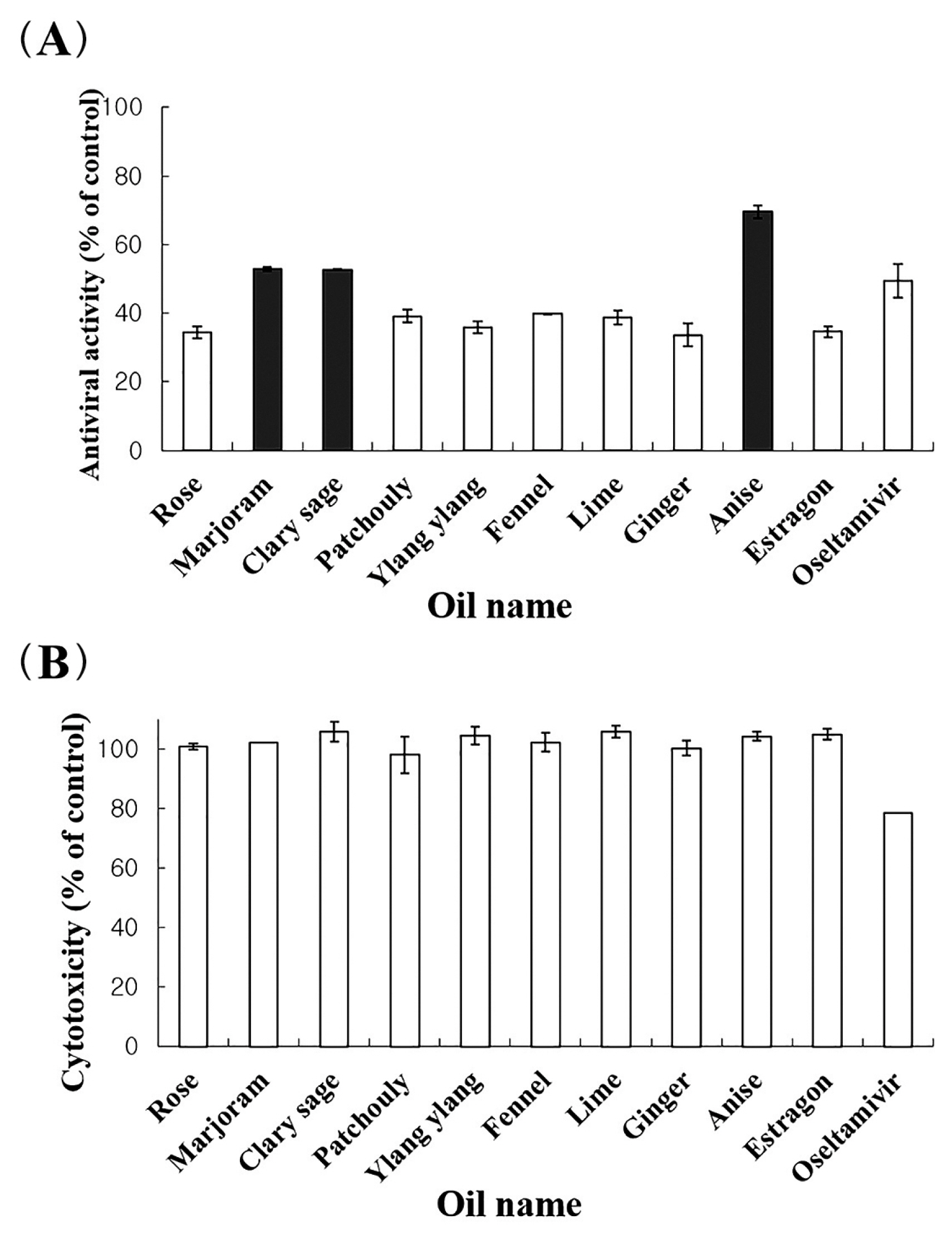

Figure 2
The effect of essential oils on influenza B/Lee/40 virus-induced CPE. The virus-infected cells were treated with essential oils (100 μg/mL). After incubation 2 days, the cells was stained by SRB and the morphology was examined.
Non-infected cells; (B) virus-infected cells without oils; (C) non-infected cells with oseltamivir; (D) virus-infected cells with oseltamivir; (E) non-infected cells with marjoram oil; (F) virus-infected cells with marjoram oil; (G) non-infected cells with clary sage oil; (H) virus-infected cells with clary sage oil; (I) non-infected cells with anise oil; (J) virus-infected cells with anise oil.
CPE = cytopathic effect; SRB = sulforhodamine B.
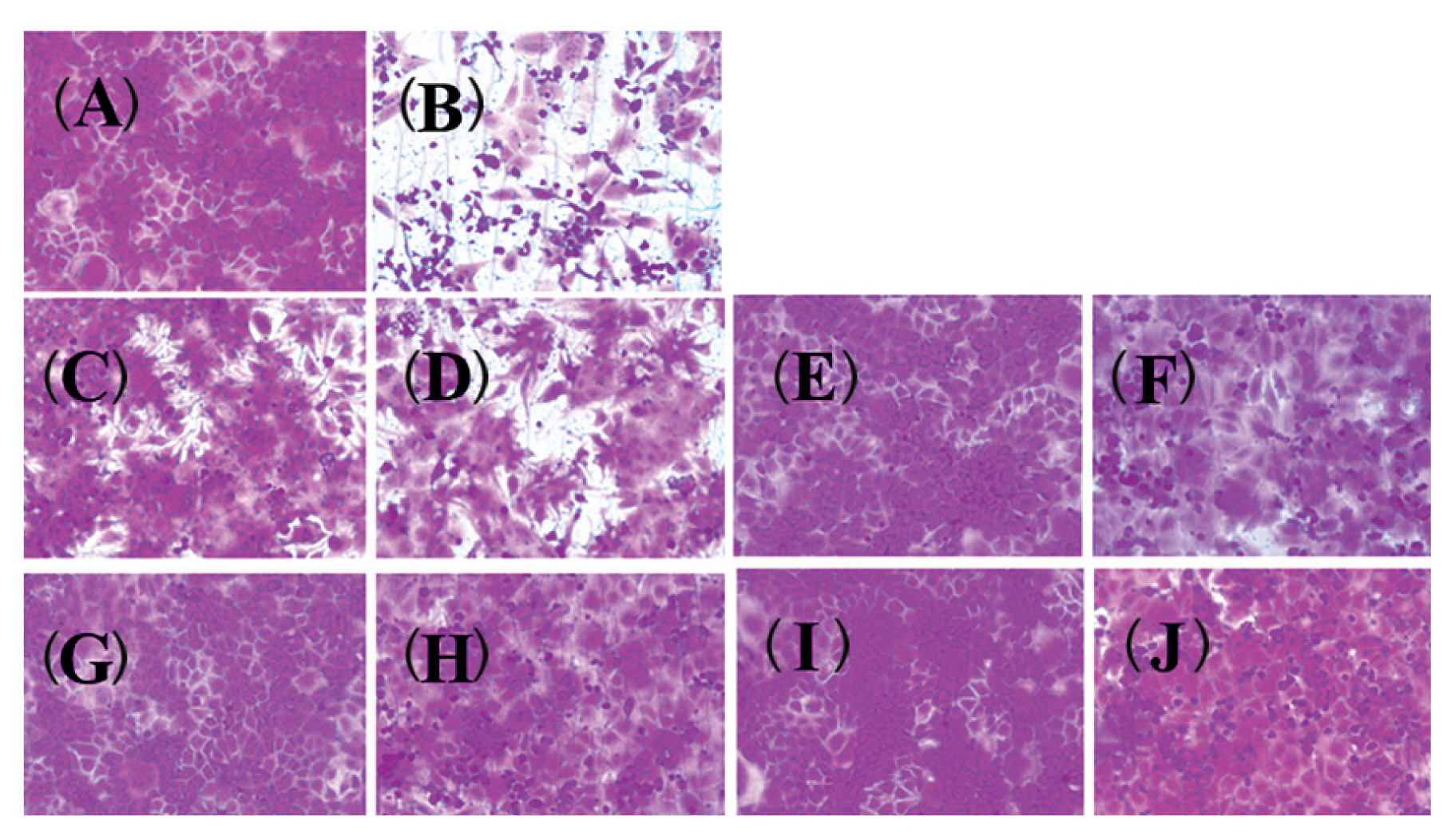

Table 1List of 62 plant essential oils tested for antiviral activity.
Table 2Chemical composition of marjoram oil.
| No. | Compound | Retention time (min) | Relative composition ratio (%) |
|---|---|---|---|
| 1 | α –pinene | 928 | 4.21 |
| 2 | Sabinene | 962 | 1.82 |
| 3 | β–pinene | 967 | 5.81 |
| 4 | β–myrcene | 981 | 1.02 |
| 5 | p-cymene | 1,010 | 0.94 |
| 6 | 1,8-cineole | 1,018 | 64.61 |
| 7 | Linalool | 1,084 | 15.28 |
| 8 | Terpinen-4-ol * | 1,159 | 1.29 |
| 9 | α–terpineol * | 1,170 | 2.40 |
| 10 | Bornylacetate * | 1,435 | 2.61 |
| Total | 100.0 |
Table 3Chemical composition of clary sage oil.
| No. | Compound | Retention time (min) | Relative composition ratio (%) |
|---|---|---|---|
| 1 | β–myrcene | 981 | 1.53 |
| 2 | Linalool | 1,084 | 22.06 |
| 3 | α–Terpineol | 1,170 | 4.21 |
| 4 | Cinnamaldehyde * | 1,234 | 1.81 |
| 5 | Linalayl acetate * | 1,240 | 61.16 |
| 6 | Geranyl acetate | 1,360 | 2.40 |
| Total | 93.17 |
Figure & Data
References
Citations
Citations to this article as recorded by 

- Phytochemistry and Biological Profile of Gaultheria procumbens L. and Wintergreen Essential Oil: From Traditional Application to Molecular Mechanisms and Therapeutic Targets
Piotr Michel, Monika Anna Olszewska
International Journal of Molecular Sciences.2024; 25(1): 565. CrossRef - A single intranasal dose of essential oil spray confers modulation of the nasopharyngeal microbiota and short-term inhibition of Mannheimia in feedlot cattle: a pilot study
Gabriela Magossi, Kaycie N. Schmidt, Thomas M. Winders, Zachary E. Carlson, Devin B. Holman, Sarah R. Underdahl, Kendall C. Swanson, Samat Amat
Scientific Reports.2024;[Epub] CrossRef - Screening and selection of essential oils for an intranasal spray against bovine respiratory pathogens based on antimicrobial, antiviral, immunomodulatory, and antibiofilm activities
Samat Amat, Gabriela Magossi, AGM Rakibuzzaman, Devin B. Holman, Kaycie N. Schmidt, Luke Kosel, Sheela Ramamoorthy
Frontiers in Veterinary Science.2024;[Epub] CrossRef - COVID-19 pandemic sheds a new research spotlight on antiviral potential of essential oils – A bibliometric study
Binawati Ginting, Williams Chiari, Teuku Fais Duta, Syihaabul Hudaa, Agnia Purnama, Harapan Harapan, Diva Rayyan Rizki, Kana Puspita, Rinaldi Idroes, Meriatna Meriatna, Muhammad Iqhrammullah
Heliyon.2023; 9(7): e17703. CrossRef - Antiviral Effect of Propylene Glycol against Envelope Viruses in Spray and Volatilized Forms
Yui Hirama, Shintaro Onishi, Ryunosuke Shibata, Hirohiko Ishida, Takuya Mori, Noriyasu Ota
Viruses.2023; 15(7): 1421. CrossRef - Essential Oils: Chemistry and Pharmacological Activities
Damião P. de Sousa, Renan Oliveira S. Damasceno, Riccardo Amorati, Hatem A. Elshabrawy, Ricardo D. de Castro, Daniel P. Bezerra, Vitória Regina V. Nunes, Rebeca C. Gomes, Tamires C. Lima
Biomolecules.2023; 13(7): 1144. CrossRef - Survey on Medicinal Plants and Herbs in Traditional Iranian Medicine
with Anti-oxidant, Anti-viral, Anti-microbial, and Anti-inflammation
Properties
Mohamad Hesam Shahrajabian, Wenli Sun
Letters in Drug Design & Discovery.2023; 20(11): 1707. CrossRef - Preventing fomite transmission using antiviral materials: Perspectives on food packaging after COVID-19 pandemic
Ruchir Priyadarshi, Shiv Dutt Purohit, Tabli Ghosh, Jong-Whan Rhim
Food Packaging and Shelf Life.2023; 40: 101171. CrossRef - In silico Molecular Docking Analysis of some Terpenoids against 3CLpro of SARS-CoV-2
Kushagra Nagori, Madhulika Pradhan, Kartik T. Nakhate, Hemant R. Badwaik, Reena Deshmukh, Ayushmaan Roy, Rashnita Sharma, Shobhit P. Srivastava, Sonia Chawla, Vishal Jain, Mukesh Sharma
Research Journal of Pharmacy and Technology.2023; : 4791. CrossRef - Antiviral and Virucidal Properties of Essential Oils and Isolated Compounds – A Scientific Approach
Jürgen Reichling
Planta Medica.2022; 88(08): 587. CrossRef - Therapeutic benefits of Salvia species: A focus on cancer and viral infection
Chinonso Anthony Ezema, Timothy Prince Chidike Ezeorba, Rita Ngozi Aguchem, Innocent Uzochukwu Okagu
Heliyon.2022; 8(1): e08763. CrossRef - Ultrastructural Damages to H1N1 Influenza Virus Caused by Vapor Essential Oils
Valentina Noemi Madia, Walter Toscanelli, Daniela De Vita, Marta De Angelis, Antonella Messore, Davide Ialongo, Luigi Scipione, Valeria Tudino, Felicia Diodata D’Auria, Roberto Di Santo, Stefania Garzoli, Annarita Stringaro, Marisa Colone, Magda Marchetti
Molecules.2022; 27(12): 3718. CrossRef - Anti-Coronavirus Efficiency and Redox-Modulating Capacity of Polyphenol-Rich Extracts from Traditional Bulgarian Medicinal Plants
Neli Vilhelmova-Ilieva, Zdravka Petrova, Almira Georgieva, Elina Tzvetanova, Madlena Trepechova, Milka Mileva
Life.2022; 12(7): 1088. CrossRef - Essential Oils and Their Compounds as Potential Anti-Influenza Agents
Ayodeji Oluwabunmi Oriola, Adebola Omowunmi Oyedeji
Molecules.2022; 27(22): 7797. CrossRef - STANDARDIZATION OF ORTHOSIPHON ARISTATUS, BLUME MIQ
FAHRAUK FARAMAYUDA, SORAYA RIYANTI, SURYANI, AKHIRUL KAHFI SYAM, ELFAHM, TOTIK MARIANI, SUKRASNO
International Journal of Applied Pharmaceutics.2022; : 72. CrossRef - Potential anti-influenza effective plants used in Turkish folk medicine: A review
Seyid Ahmet Sargin
Journal of Ethnopharmacology.2021; 265: 113319. CrossRef - ‘BhAVI-23’-A spice-herb based dietary infusion possessing in-vitro anti-viral potential
Sudhanshu Saxena, Sanjeev Kumar, Sachin N. Hajare, Sumit Gupta, Satyendra Gautam, Sunil K. Ghosh
Journal of Ayurveda and Integrative Medicine.2021; 12(2): 312. CrossRef - Non-Cannabinoid Metabolites of Cannabis sativa L. with Therapeutic Potential
Henry Lowe, Blair Steele, Joseph Bryant, Ngeh Toyang, Wilfred Ngwa
Plants.2021; 10(2): 400. CrossRef - Antiviral activity of Lavandula angustifolia L. and Salvia officinalis L. essential oils against avian influenza H5N1 virus
Doha H. Abou Baker, Ryszard Amarowicz, Ahmed Kandeil, Mohamed A. Ali, Eman A. Ibrahim
Journal of Agriculture and Food Research.2021; 4: 100135. CrossRef - In vitro Assessment of Antiviral Effect of Natural Compounds on Porcine Epidemic Diarrhea Coronavirus
Manuel Gómez-García, Héctor Puente, Héctor Argüello, Óscar Mencía-Ares, Pedro Rubio, Ana Carvajal
Frontiers in Veterinary Science.2021;[Epub] CrossRef - Natural oil blend formulation as an anti-African swine fever virus agent in in vitro primary porcine alveolar macrophage culture
Quang Lam Truong, Lan Thi Nguyen, Haig Yousef Babikian, Rajeev Kumar Jha, Hoa Thi Nguyen, Thanh Long To
Veterinary World.2021; 14(3): 794. CrossRef - Investigative study into whether an insect repellent has virucidal activity against SARS-CoV-2
Sophie J. Smither, Lin S. Eastaugh, James S. Findlay, Thomas R. Laws, Stephen N. Marriott, Stuart Notman, Lyn M. O’Brien, Amanda L. Phelps, Mark Richards, David Ulaeto, Pat Watts, Mark S. Lever, Norman Govan
Journal of General Virology .2021;[Epub] CrossRef - Transdermal Film Loaded with Garlic Oil-Acyclovir Nanoemulsion to Overcome Barriers for Its Use in Alleviating Cold Sore Conditions
Alshaimaa M. Almehmady, Sarah A. Ali
Pharmaceutics.2021; 13(5): 669. CrossRef - Protective Action of L. salivarius SGL03 and Lactoferrin against COVID-19 Infections in Human Nasopharynx
Marzena Kucia, Ewa Wietrak, Mateusz Szymczak, Michał Majchrzak, Paweł Kowalczyk
Materials.2021; 14(11): 3086. CrossRef - Novel formulation with essential oils as a potential agent to minimize African swine fever virus transmission in an in vivo trial in swine
Haig Yousef Babikian, Rajeev Kumar Jha, Quang Lam Truong, Lan Thi Nguyen, Yusef Babikyan, Hoa Thi Nguyen, Thanh Long To, Ali Agus
Veterinary World.2021; : 1853. CrossRef - Effect of hot air and infrared drying on the retention of cannabidiol and terpenes in industrial hemp (Cannabis sativa L.)
Chang Chen, Ivan Wongso, Daniel Putnam, Ragab Khir, Zhongli Pan
Industrial Crops and Products.2021; 172: 114051. CrossRef - Cinnamon and its possible impact on COVID-19: The viewpoint of traditional and conventional medicine
Maryam Yakhchali, Zahra Taghipour, Mehran Mirabzadeh Ardakani, Mahdi Alizadeh Vaghasloo, Mahdi Vazirian, Sima Sadrai
Biomedicine & Pharmacotherapy.2021; 143: 112221. CrossRef - Evaluation of botanicals as potential COVID-19 symptoms terminator
Ufuk Koca Caliskan, Methiye Mancak Karakus
World Journal of Gastroenterology.2021; 27(39): 6551. CrossRef - Antiviral Activities of Eucalyptus Essential Oils: Their Effectiveness as Therapeutic Targets against Human Viruses
Daniel Mieres-Castro, Sunny Ahmar, Rubab Shabbir, Freddy Mora-Poblete
Pharmaceuticals.2021; 14(12): 1210. CrossRef - Cannabidiol and terpenes from hemp – ingredients for future foods and processing technologies
Chang Chen, Zhongli Pan
Journal of Future Foods.2021; 1(2): 113. CrossRef - Natural Products, a Potential Therapeutic Modality in Management and Treatment of nCoV-19 Infection: Preclinical and Clinical Based Evidence
Ashif Iqubal, Mohammad K. Iqubal, Musheer Ahmed, Syed E. Haque
Current Pharmaceutical Design.2021; 27(9): 1153. CrossRef - New Tricks for Old Guys: Recent Developments in the Chemistry, Biochemistry, Applications and Exploitation of Selected Species from the Lamiaceae Family
Edoardo Napoli, Laura Siracusa, Giuseppe Ruberto
Chemistry & Biodiversity.2020;[Epub] CrossRef - Chemistry, bioactivities, mode of action and industrial applications of essential oils
B. Sharmeen Jugreet, Shanoo Suroowan, R.R. Kannan Rengasamy, M. Fawzi Mahomoodally
Trends in Food Science & Technology.2020; 101: 89. CrossRef - Exploring multiple mechanisms of Qingjie Fanggan prescription for prevention and treatment of influenza based on systems pharmacology
Kai Gao, Yan-Ping Song, Xia Du, Hao Chen, Lin-Tao Zhao
Computational Biology and Chemistry.2020; 88: 107307. CrossRef - Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data
Razina Rouf, Shaikh Jamal Uddin, Dipto Kumer Sarker, Muhammad Torequl Islam, Eunus S. Ali, Jamil A. Shilpi, Lutfun Nahar, Evelin Tiralongo, Satyajit D. Sarker
Trends in Food Science & Technology.2020; 104: 219. CrossRef - Antiviral Effects of Plant-Derived Essential Oils and Their Components: An Updated Review
Li Ma, Lei Yao
Molecules.2020; 25(11): 2627. CrossRef - Thymus mastichina: Composition and Biological Properties with a Focus on Antimicrobial Activity
Márcio Rodrigues, Ana Clara Lopes, Filipa Vaz, Melanie Filipe, Gilberto Alves, Maximiano P. Ribeiro, Paula Coutinho, André R. T. S. Araujo
Pharmaceuticals.2020; 13(12): 479. CrossRef


 PubReader
PubReader Cite
Cite