Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 5(Suppl); 2014 > Article
-
Original Article
Utilization of Laboratory Tests for Tuberculosis and Mycobacterial Disease in Korea - Chang-Ki Kima, Sung Won Choia, Mi-Sun Parkb
-
Osong Public Health and Research Perspectives 2014;5(Suppl):S24-S29.
DOI: https://doi.org/10.1016/j.phrp.2014.10.008
Published online: November 4, 2014
aKorean Institute of Tuberculosis, Cheongju, Korea
bDivision of Tuberculosis and Bacterial Respiratory Infections, Korea National Institute of Health, Cheongju, Korea
- ∗Corresponding author. psoas95@gmail.com
• Received: October 15, 2014 • Revised: October 26, 2014 • Accepted: October 27, 2014
© 2014 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the CC-BY-NC License (http://creativecommons.org/licenses/by-nc/3.0).
Abstract
-
Objectives
- In Korea, a large portion of tuberculosis (TB) patients are diagnosed and treated in private institutes. Laboratory tests are crucial for TB control. There are many possible problems using laboratory tests in the private sector. In this study, we aimed to investigate the characteristics and trends of utilizing laboratory tests for TB and mycobacterial diseases in the private sector by analyzing the National Health Insurance (NHI) database.
-
Methods
- After selecting TB or other mycobacteria-related test items, we searched the number and cost of each item on the website of the Health Insurance Review and Assessment Service using the code of each test from 2007 to 2012.
-
Results
- Our data revealed that the number and cost of tests drastically increased between 2007 and 2012. Culture and molecular tests primarily contributed to the tremendous increases. For each year, concentrated smearing and fluorochrome staining were more commonly used. The number of serologic tests for latent TB infection stagnated, despite the expansion of contact investigation.
-
Conclusion
- The NHI data could be considerably useful for understanding the utilization trends of laboratory tests for TB and mycobacterial diseases in Korea. Our data showed that TB laboratory systems have recently improved. In this study, many issues were noticed. Therefore, solutions to these issues are required and the continued monitoring of NHI data regarding laboratory diagnosis.
- Tuberculosis (TB) is a very serious health threat in the world [1]. Many people develop and die of TB. Diagnosis and treatment are important pillars for controlling and eradicating TB, and laboratory tests are crucial for its diagnosis. The roles of TB laboratory tests are to detect patients with TB, determine drug susceptibility, and monitor treatment response. Several conventional tests have been used for almost a century. However, there have been tremendous changes in TB diagnosis, and many new diagnostics have been introduced and implemented for TB control [2]. Therefore, standardized use of various laboratory tests is necessary. The World Health Organization (WHO) released guidelines or policy statements regarding diagnosis to help TB programs use laboratory tests properly and adopt optimal diagnostic processes. Many countries, including Korea, prepare their own national guidelines for diagnosis and enable health workers to follow these guidelines [3]. However, this approach usually works well only in the public sector, not the private sector. There are many problems in the private sector in following a standardized practice. According to a TB notification report from the Korea Centers for Disease Control and Prevention (KCDC), >90% of patients with TB went to private institutes [4]. There are presumably many problems in using TB tests in the private sector, but few problems have been revealed. South Korea has a National Health Insurance (NHI) system, which is required by Korean law. It manages all medical practices and every private institute claims the fee of medical practice from NHI system. In this study, we aimed to investigate the characteristics and trends in the utilization of laboratory tests for TB and mycobacterial diseases in the private sector by analyzing the NHI database.
Introduction
- We analyzed the insurance data of laboratory tests in relation to TB and mycobacterial diseases from 2007 to 2012. The names and insurance codes of the tests were selected from the health insurance database. These are listed in Table 1 and included smear microscopy, culture, drug susceptibility testing (DST), nucleic acid amplification test (NAAT), rapid DST, identification of nontuberculous mycobacteria (NTM), and serologic tests for latent TB infection. We searched utilization information and the number of tests and their cost on the website of the Health Insurance Review and Assessment Service by using the code of each test (http://hira.or.kr/rdd_disease.do?method=listInfoMdfee&pgmid=HIRAA020044020200).
Materials and methods
- The total number of tests performed in 2007 was 1,941,086, which increased to 3,083,491 in 2012 (Table 1; Figure 1). The cost for TB and NTM laboratory tests was approximately 200 million Korean won, but it increased to more than twice this amount in 2012 (Figure 2).
- 3.1 Conventional tests 3.1.1
- Smear microscopy was the most frequently used test item for TB and NTM disease. In 2007, smear microscopy accounted for 56.2% of all tests. Since then, the proportion gradually declined and culture examination exceeded smear microscopy. Although the tested number was very large, its cost accounted for 13% in 2012. Smear microscopy had four types of smear preparation and straining methods. The proportion of concentrated smear tests with fuschin staining was highest in 2007; thereafter, it gradually decreased. In 2012, concentrated smear with fluorochrome staining was the most frequently performed test. 3.1.2
- There are two test codes for culture examination based on media type. The number of solid cultures gradually increased during 2007–2012. Liquid culture has been covered by health insurance since 2008, before which only 15,736 liquid cultures were tested. However, the number of liquid cultures thereafter rapidly increased. In 2012, solid cultures and liquid cultures were tested for 844,729 samples and 558,094 samples, respectively. In 2012, culture examinations accounted for 47.7% of all costs. Therefore, culture examination ranked first in number and in cost in 2012. 3.1.3
- The number of drug susceptibility tests (DSTs) increased gradually during 2007–2012. There are two codes for DST based on the number of drugs tested. However, in 2012, DST (for <10 drugs) was not commonly used and its proportion was only 3.6% (1,427/39,987 tests). During 2007–2012, there was no increasing or decreasing trend in the use of DST (for <10 drugs). Most DSTs were performed for 10 or more drugs.
- 3.2 Molecular tests 3.2.1
- The number of TB NAATs dramatically increased during 2007–2014. A total of 140,037 NAATs were performed in 2007, but the tested number more than doubled to 294,761 tests in 2012. The cost for NAAT was 15,111,149 Korean won, which was the second highest cost in 2012. Three methods were used for TB NAAT in Korea: TB polymerase chain reaction (PCR), TB nested PCR, and TB real-time PCR. TB PCR was the most simple test and not widely used. TB nested PCR was more commonly used, compared to TB PCR, but the number of tests stagnated during 2007–2012. TB real-time PCR was the most commonly used TB NAAT. 3.2.2
- Rapid DST was available for rifampicin (RIF) and isoniazid in Korea. Rapid DST was performed for only 26,330 samples in 2007 and for 13,138 samples in 2012. The number of RIF and isoniazid tests were very similar, which indicates that most laboratories tested two drugs at the same time. 3.2.3
- NTM identification tests numbered more than 9000 tests in 2009. The number of NTM identification tests during 2010–2011 stagnated without any significant change. However, in 2012, it suddenly declined to 6190 tests.
- 3.3 Serologic tests for latent TB infection 3.3.1
- A total of 34,467 tuberculin skin tests (TSTs) were performed in 2007. Since then, the number of TSTs stagnated. The proportion of TST was very small in number and in cost. 3.3.2
- The NHI system started covering interferon-gamma release assay (IGRA) in 2009. Therefore, 3 years of data were analyzed. In the 1st year, 5468 IGRAs were performed. There was no significant change or increasing trend in the utilization of IGRA during the first 3 years. However, the number of IGRAs performed in 2012 increased to 6803 tests.
Results
3.1.1 Smear microscopy
3.1.2 Culture examination
3.1.3 DST
3.2.1 NAAT
3.2.2 Rapid DST
3.2.3 NTM identification
3.3.1 Tuberculin skin test
3.3.2 Interferon-gamma release assay
- Standardized diagnosis and treatment are important for successful TB control. Therefore, the KCDC published Korean guidelines for TB in 2011, which was intended to help physicians follow the standards. The guidelines confirmed that physicians should use laboratory tests for diagnosis of TB with chest x-ray [3]. The recommendations of the guidelines include performing cultures for every sample requested, using liquid cultures along with solid media, and determining drug susceptibility for all culture-positive TB patients and rapid DST for multidrug-resistant TB (MDR-TB) suspects [3]. These recommendations are very important for the diagnosis and detection of drug-resistant TB.
- Our results showed many problems in using laboratory tests. Essential tests were often neglected. In 2007, more than one million smear examinations were performed, but only 58.8% of them were cultured. The number of RIF rapid DSTs performed in 2007 was 1565 tests, which was only 6.0% of DST.
- Liquid culture has many advantages over solid culture [5]. It detects more TB cases in a shorter period of time. Therefore, the WHO approves liquid culture as the standard culture method [6]. However, liquid culture had not been widely used in Korea because the insurance fee for this culture is too low for laboratories to perform liquid culture and there is no specific insurance code for liquid culture. However, much improvement occurred during 2007–2012. First, the proportion of concentrated smear and fluorochrome staining increased. In 2007, smear examination using concentration accounted for 60% of all smears. The proportion of smears using the concentration method increased to 69.6% in 2012. The proportion of fluorochrome staining dramatically increased from 37.1% to 65.0% during that period. According to Korean guidelines for TB, every sample for which TB testing is requested should be cultured using both liquid media and solid media. As previously described, liquid culture has seldom been used. In 2008, when liquid culture was approved by NHI, only 15,736 samples were cultured with liquid media. However, the number of liquid cultures rapidly increased since then. In 2012, more than half a million liquid cultures were performed, which is approximately two-thirds of the number of solid cultures. Most samples presumably will soon be cultured using both types of media. Rapid DST is necessary for the timely detection of MDR-TB [3,7]. Therefore, MDR-TB suspects who should undergo rapid DST include MDR contacts, patients with a previous TB treatment history, and patients with treatment failure. Initially, rapid DST was performed for a few TB patients. However, the number of rapid DSTs performed increased by more than four times from 2007 to 2012. Xpert MTB/RIF (Xpert, Cepheid, Sunnyvale, CA) was not included for analysis because Xpert was not approved by the KCDC until 2013. The introduction of Xpert will influence the utilization of rapid DST. Therefore, careful monitoring must be followed.
- There were other interesting findings from our analysis. Guidelines recommend determining the susceptibility to first-line drugs for new TB cases and performing DST of second-line drugs for TB cases resistant to first-line drugs [3,7–9]. However, most DST was performed for more than 10 drugs, and most TB cases seem to be tested by DST of first- and second-line drugs simultaneously. This may be because testing a few drugs is less profitable to a laboratory. The insurance fee for DST (for <10 drugs) is unreasonably low, considering the expense of the equipment, facility, human resources, and reagents. The number of DSTs had been gradually increasing, but drastic increase was noticed in 2012. The DST code actually covers both anti-TB testing and NTM testing. NTM diseases are increasingly diagnosed and more tests for NTM are being conducted in Korea. We assumed that NTM DST may contribute to the rapid increase in the number of drug susceptibility tests. However, NTM identification was performed less in 2012 than in previous years, despite an increase in the incidence of NTM disease. The insurance code (CY636) for NTM identification specifically indicates the PCR-restriction enzyme analysis method. Line probe assay has been used more commonly for NTM identification compared to the PCR-restriction enzyme analysis method. Laboratories may use the code (C6021) for PCR hybridization (i.e., real-time PCR), which is similar to the line probe assay because there is no specific insurance code for NTM identification using other methods. Even though the KCDC has been trying hard to expand contact investigation, the number of TSTs and IGRAs stagnated between 2007 and 2012. It was partially because of this that physicians were passive or reluctant to investigate contacts. Most investigations are also conducted by the central or local government.
- The total number and cost of laboratory tests drastically increased from 2007 to 2012. However, there had been no significant change in TB notification during the same period. It is difficult to explain what influenced the trend in test utilization in the private sector. We nonetheless assumed that the causes could be expanded coverage of new diagnostics, improved physician awareness, introduction of standardized TB guidelines, and increased prevalence of NTM diseases.
- This study has several limitations. Institutes may claim some tests by using the wrong insurance codes. Some insurance codes can be used for different tests. In that situation, it is impossible to collect information about every test through the NHI data. Furthermore, the NHI system officially specifies nonpayment services and allows the patient to cover all their costs. The NHI system has no record of these services; therefore, this study was unable to include nonpayment tests.
- In conclusion, the NHI data would be very useful for understanding trends in the utilization of laboratory tests for TB and mycobacterial diseases in Korea. Our data showed that TB laboratory systems have improved recently. In this study, many issues were also noticed. Therefore, solutions to these issues need to be found and monitoring of NHI data concerning laboratory diagnosis needs to continue.
Discussion
- All contributing authors declare no conflicts of interest.
Conflicts of interest
-
Acknowledgements
- This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (Cheongju, Korea) (2013-E46003-00).
Acknowledgments
-
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article information
- 1. World Health Organisation (WHO) . Global tuberculosis control: WHO report 2012. 2013. WHO; Geneva, Switzerland: [in press].
- 2. Kim C.K., Sung H., Park Y.J.. A proposal for laboratory workflow changes for efficient tuberculosis control. Ann Clin Microbiol 16(2). 2013 Jun;61−68.Article
- 3. Korea Centers for Disease Control and Prevention (KCDC) . Korean guidelines for tuberculosis. 2011. KCDC; Cheongju, Korea.
- 4. Korea Centers for Disease Control and Prevention (KCDC) . Annual report on the notified tuberculosis in Korea. 2013. KCDC; Cheongju, Korea.
- 5. Clinical and Laboratory Standards Institute (CLSI) . Laboratory detection and identification of mycobacteria; approved guideline, M48-A. CLSI document M48-A. 2008. CLSI; Wayne, PA.
- 6. World Health Organization (WHO) . Use of liquid TB culture and drug susceptibility testing (DST) in low and medium income settings. 2007. WHO; Geneva, Switzerland.
- 7. World Health Organization (WHO) . Guidelines for the programmatic management of drug-resistant tuberculosis. 2011. WHO; Geneva, Switzerland.
- 8. Clinical and Laboratory Standards Institute (CLSI) . Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard—2nd edition. CLSI document M24–A2. 2011. CLSI; Wayne, PA.
- 9. World Health Organization (WHO) . Guidelines for treatment of tuberculosis. 4th ed.2010. WHO; Geneva, Switzerland.
References
Figure 1Requested number of laboratory tests for tuberculosis and mycobacterial diseases for 2007–2012. The total number of tests increased gradually in this period. Smear microscopy and culture examinations have the greatest number of tests. DST = drug susceptibility testing; LTBI = latent tuberculosis infection; NAAT = nucleic acid amplification test; NTM ID = nontuberculous mycobacteria identification.
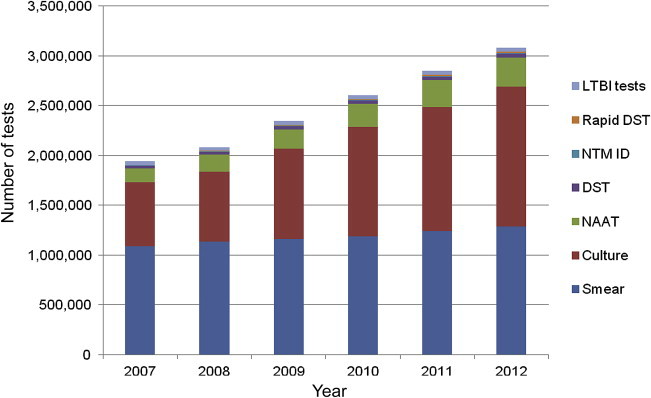

Figure 2The cost of laboratory tests for tuberculosis and mycobacterial diseases during 2007–2012. The total cost for laboratory tests rapidly increased during this period. This increase is primarily because of increased utilization of NAAT and culture examination. Smear is the most frequently requested test, but the proportion of smear microscopy is relatively low in cost. DST = drug susceptibility testing; LTBI = latent tuberculosis infection; NAAT = nucleic acid amplification test; NTM ID = nontuberculous mycobacteria identification.
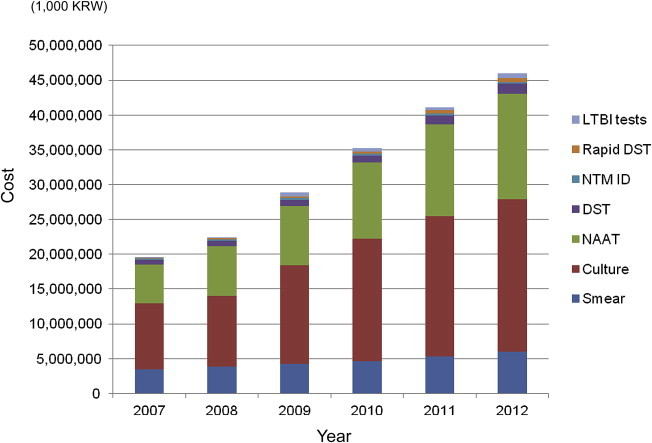

Table 1Insurance codes and tested number of laboratory tests for TB and mycobacterial disease (2007–2012).
Figure & Data
References
Citations
Citations to this article as recorded by 

- Tuberculosis Surveillance and Monitoring under the National Public-Private Mix Tuberculosis Control Project in South Korea 2016–2017
Jinsoo Min, Hyung Woo Kim, Yousang Ko, Jee Youn Oh, Ji Young Kang, Joosun Lee, Young Joon Park, Sung-Soon Lee, Jae Seuk Park, Ju Sang Kim
Tuberculosis and Respiratory Diseases.2020; 83(3): 218. CrossRef - Is Tuberculosis Still the Number One Infectious Disease in Korea?
Hae-Wol Cho, Chaeshin Chu
Osong Public Health and Research Perspectives.2014; 5: S1. CrossRef


 PubReader
PubReader Cite
Cite
