Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 9(6); 2018 > Article
-
Original Article
Effects of Injection Laryngoplasty with Hyaluronic Acid in Patients with Vocal Fold Paralysis - Geun-Hyo Kima, Jae-Seok Leeb, Chang-Yoon Leec, Yeon-Woo Leea, In-Ho Baed, Hee-June Parke, Byung-Joo Leea, Soon-Bok Kwonf
-
Osong Public Health and Research Perspectives 2018;9(6):354-361.
DOI: https://doi.org/10.24171/j.phrp.2018.9.6.10
Published online: December 31, 2018
aDepartment of Otorhinolaryngology-Head and Neck Surgery and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
bDepartment of Otolaryngology, Gyeongsang National University Hospital, Jinju, Korea
cDepartment of Otorhinolaryngology, Dongnam Institute of Radiological & Medical Sciences, Busan, Korea
dDepartment of Otorhinolaryngology-Head and Neck Surgery, Pusan National University Yangsan Hospital, Yangsan, Korea
eDepartment of Speech Rehabilitation, Choonhae College of Health Sciences, Ulsan, Korea
fDepartment of Humanities, Language and Information, Pusan National University, Busan, Korea
- *Corresponding author: Soon-Bok Kwon, Department of Humanities, Language and Information, Pusan National University, Pusan, Korea, E-mail: voicesbkwon@gmail.com
Copyright ©2018, Korea Centers for Disease Control and Prevention
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- The purpose of this study was to explore the effects of injection laryngoplasty (IL) with hyaluronic acid in patients with vocal fold paralysis (VFP).
-
Methods
- A total of 50 patients with VFP participated in this study. Pre- and post-IL assessments were performed, which included analyzing the sustained vowel /a/ phonation, and the patient reading 1 Korean sentence from the “Walk” passage that comprised 25 syllables in 10 words. To investigate the effect of IL on vocal fold function, acoustic analysis (acoustic voice quality index, cepstral peak prominence, maximum phonation time, speaking fundamental frequency) was conducted and auditory-perceptual (grade and overall severity), visual judgment (gap), and self-questionnaire (voice handicap index-10) assessments were performed.
-
Results
- The patients with VFP showed statistically significant differences between pre-and post-IL assessments for acoustic and auditory-perception, visual judgment, and self-questionnaire assessments.
-
Conclusion
- The patients with VFP showed positive change in vocal fold function between pre- and post-IL measurements. The findings showed that IL with hyaluronic acid is an effective method to improve vocal fold function in patients with VFP.
- Vocal fold paralysis (VFP) is defined as the immobility of the vocal fold due to the disruption of its motor innervation [1]. In the adult population, VFP most commonly arises from recurrent laryngeal nerve injury due to cardiac, neck, and mediastinal surgery, as well as from neurologic and idiopathic causes [2]. Patients with VFP often have complaints of cough and a hoarse, weak, or breathy voice [3]. Surgical interventions for VFP include injection laryngoplasty (IL), surgical medialization, and laryngeal reinnervation [4]. Surgical interventions are ideally performed in conjunction with preoperative and postoperative voice therapy. Among these interventions, IL is a minimally invasive method used for the treatment of VFP [5]. Glottal closure can be improved by injecting various substances into the paralyzed fold, which include human acellular tissue matrix, autologous fat, autologous fascia, calcium hydroxylapatite, hyaluronic acid (HA), gel implants, and other implants that are currently available in clinical practice for IL [6]. Appropriate application of these substances may improve vocal fold function. Among these, HA derivatives are one of the most promising substances currently under investigation in human studies. Long-term follow-up observations have been conducted to determine their safety and efficacy [7].
- Various assessments can be performed to evaluate vocal fold function. A voice quality evaluation is conducted by completing various voice assessment forms after surgical intervention [8]. Multidimensional voice evaluations in patients with VFP include self-questionnaires and acoustic, auditory-perceptual, and visual assessments. An acoustic voice evaluation is a non-invasive and easy way to measure the voice quality and confirm the effectiveness of IL treatment on vocal fold function. Acoustic analysis involves quantitative measurements, including perturbation and cepstral analyses. Generally, perturbation analysis (jitter, shimmer, and speaking fundamental frequency), spectral analysis (noise to harmonic ratio), cepstral analysis [cepstral peak prominence (CPP), the mean ratio of signal energy below 4,000 Hz to the energy above 4,000 Hz (L/H spectral ratio)], and maximum phonation time (MPT) are used, but the sustained vowel and connected sentence methods are often analyzed separately. The pathology of the patient’s voice should be analyzed by combining the results of the sustained vowel method and other extended vocal tasks.
- The acoustic voice quality index (AVQI) was introduced for this purpose [9]. This study used AVQI to demonstrate the effectiveness of IL. The conventional method does not reliably measure very severe voice impairment before treatment with IL. Therefore, there was a limitation in quantitatively and accurately proving the effect of IL; however, this was overcome by utilizing AVQI measurements. In this study, the AVQI was measured along with the conventional evaluation method to confirm the effect of IL. AVQI can be used to evaluate dysphonia severity, by analyzing samples that concatenate sustained vowel phonations and continuous speech. AVQI is estimated using a specific algorithm that weighs 6 variables (cepstral peak prominence smoothed (CPPS); harmonics-to-noise ratio; shimmer local; shimmer local dB; the slope and tilt of the regression line through the long-term average spectrum). This measurement is automatically obtained using the Praat script (Institute of Phonetic Sciences, University of Amsterdam, The Netherlands). A number of studies have investigated the effect of IL in patients with VFP [10–13]. However, there is currently no study that reports using AVQI analysis to evaluate the effectiveness of IL treatment. The auditory-perceptual assessment uses the GRBAS scale (grade, rough, breathy, asthenic, strained) and the Consensus Auditory-Perceptual Assessment of Voice (CAPE-V) protocol. The GRBAS scale gives ratings from 0 to 3, in which 0 is normal, 1 is mild dysphonia, 2 is moderate dysphonia, and 3 is severe dysphonia [14]. The CAPE-V provides a visual analogue scale of 100 mm, with an anchor point. Previous studies have reported a high correlation between the AVQI and auditory perceptual assessment [15–17]. Glottal gap is determined by using the method presented in the previous study [18]. Incomplete glottal closure is related to the dysphonia in VFP patients. Glottal gap was assessed using a laryngeal video endoscopy, and this visual judgment was used as the gold standard [19]. The voice handicap index (VHI)-10 demonstrated, that with 10 questions selected from the original VHI that had 30 questions, the same power with previous studies was achieved [20].
- In this study, the effect of IL (with HA) in patients with VFP was evaluated by examining changes in acoustic, auditory-perceptual, and visual judgment measurements and in the self-questionnaire responses.
Introduction
- 1. Participants
- Fifty patients (33 males and 17 females) with VFP participated in this study. The cases of VFP in these patients were attributed to idiopathic causes (n = 25), thoracic surgery (n = 14), thyroid surgery (n = 4), lung cancer (n = 3), infection (n = 3), and recurrent laryngeal nerve invasion (n = 1). There were 33 cases of VFP on the left side, and 17 on the right side. In all 50 patients, the injection material was HA. IL was performed by transcutaneous injection in the operation room. The amount of HA used for injection was determined by an experienced otolaryngologist (> 20 years experience).
- The characteristics of the research patients are shown in Table 1. The clinical diagnoses were based on clinical evaluations using laryngoscopy, laryngeal video stroboscope, computerized tomography, and laryngeal electromyography. The Institutional Review Board of Pusan National University Hospital approved this study (H-1807-019-069).
- 2. Voice recording procedure
- One hour prior to IL, the voice of each patient was recorded using a voice recording system (Computerized Speech Lab, CSL Model 4500, KayPENTAX, Lincoln Park, NJ, USA). Recordings were made of (1) the sustained vowel /a/ phonation at a comfortable and habitual pitch and loudness, and (2) the patient reading one Korean sentence from the “Walk” passage, which comprised 25 syllables in 10 words: “neolbge pyeolchyeoissneun badaleul balabomyeon nae ma-eum yeogsi neolb-eojineun geos gatda.” In accordance with the International phonetic alphabet, this passage is pronounced as [nʌlg*e pʰyʌltsʰʌinnɯn badarɯl parabomyʌn nɛ maɯm yʌk̚si nʌlbʌdzinɯn kʌt̚ kat̚t*a]. The central 2 seconds of the sustained vowel /a/ phonation and the “Walk” passage were concatenated by Praat (Version 5.4. 19) for AVQI analysis. As a consequence, the voice sample corpus included concatenated files comprising phonations of sustained vowels and a reading of the “Walk” passage from each patient. A voice recording was performed at 4 weeks post-IL to assess voice recovery.
- 3. Acoustic analysis
- The Praat script of Maryn et al [21] was modified for acoustic analysis. AVQI performs acoustic analysis designed to quantify the severity of dysphonia through assessing the phonations of sustained vowels, and reading a connected sentence. Pathological voices have larger AVQI values than normal voices. CPPS is a measurement of the relative amplitude of the cepstral peak prominence in relation to the expected amplitude as derived via linear regression. This measurement reflects the degree of regularity or periodicity in the voice signal. Higher values reflect greater periodicity [22]. MPT is the longest period during which a patient can sustain phonation of a vowel sound, typically /a/ [23]. Speaking fundamental frequency is the central tendency of the frequency of vibration of the vocal folds during connected speech, and correlates with the perceived pitch of a speaker’s voice [24] auto”/><w:left w:val=”none” w:sz=”0” w:space=”0” w:color=”auto”/><w:bottom w:val=”non.
- 4. Auditory-perceptual assessment, visual judgment, and VHI-10
- Three speech-language pathologists, all native Koreans with more than 10 years of experience in voice therapy and dysphonia ratings, were asked to rate voice samples; 2 of the speech-language pathologists worked in ENT clinics and 1 was a professor specializing in voice and swallowing disorders. Grade was evaluated for dysphonia severity using the Japan Society of Logopedics and Phoniatrics methods, and was based on a 4-point ordinal scale (normal: 0; mild: 1; moderate: 2; severe: 3) [25]. Overall severity (OS), was evaluated based on the CAPE-V method, which uses a visual analog scale of 100 mm, with anchoring points, as suggested by the Special Interest Division 3, Voice and Voice Disorders of the American Speech-Language-Hearing Association [26]. The evaluators were blinded to the pre-IL and post-IL voice samples. The recordings of 25 randomly selected patients (50% of judged voice samples taken from the first visit) were evaluated after a second visit (at 4 weeks post-IL) to calculate the intra-rater variability. Ratings of the auditory-perceptual assessments between different raters were compared to estimate the inter-rater variability.
- Visual judgment confirmed the glottal configuration in each case of VFP (median: 0, paramedian: 1, intermediate: 2, and fully abducted: 3) [18].
- The VHI-10 was created by selecting the 10 most robust VHI items determined from item analysis and clinical consensus results [20]. The patients were asked to complete the VHI-10 questionnaire before and after IL to evaluate their perception of their vocal handicap.
- 5. Statistical analysis
- All statistical analyses were completed using R version 3.4.1 (The R Foundation for Statistical Computing, Vienna, Austria) and RStudio 1.0.143 (RStudio Inc., Boston, MA, USA). Paired t test was used to perform comparisons between pre- and post-IL measurements for all patients. Pearson correlation coefficient (rp) was used to investigate the correlation among all variables. For data analysis and output, R package libraries such as ggplot2, ggsignif, and corrplot were used.
Materials and Methods
- 1. Rater variability
- For auditory-perceptual and visual judgment assessments, the inter-rater reliability ranged from 0.71 to 0.90, while the intra-rater reliability ranged from 0.83 to 0.92.
- 2. Comparison of all variables from pre- and post-IL assessments
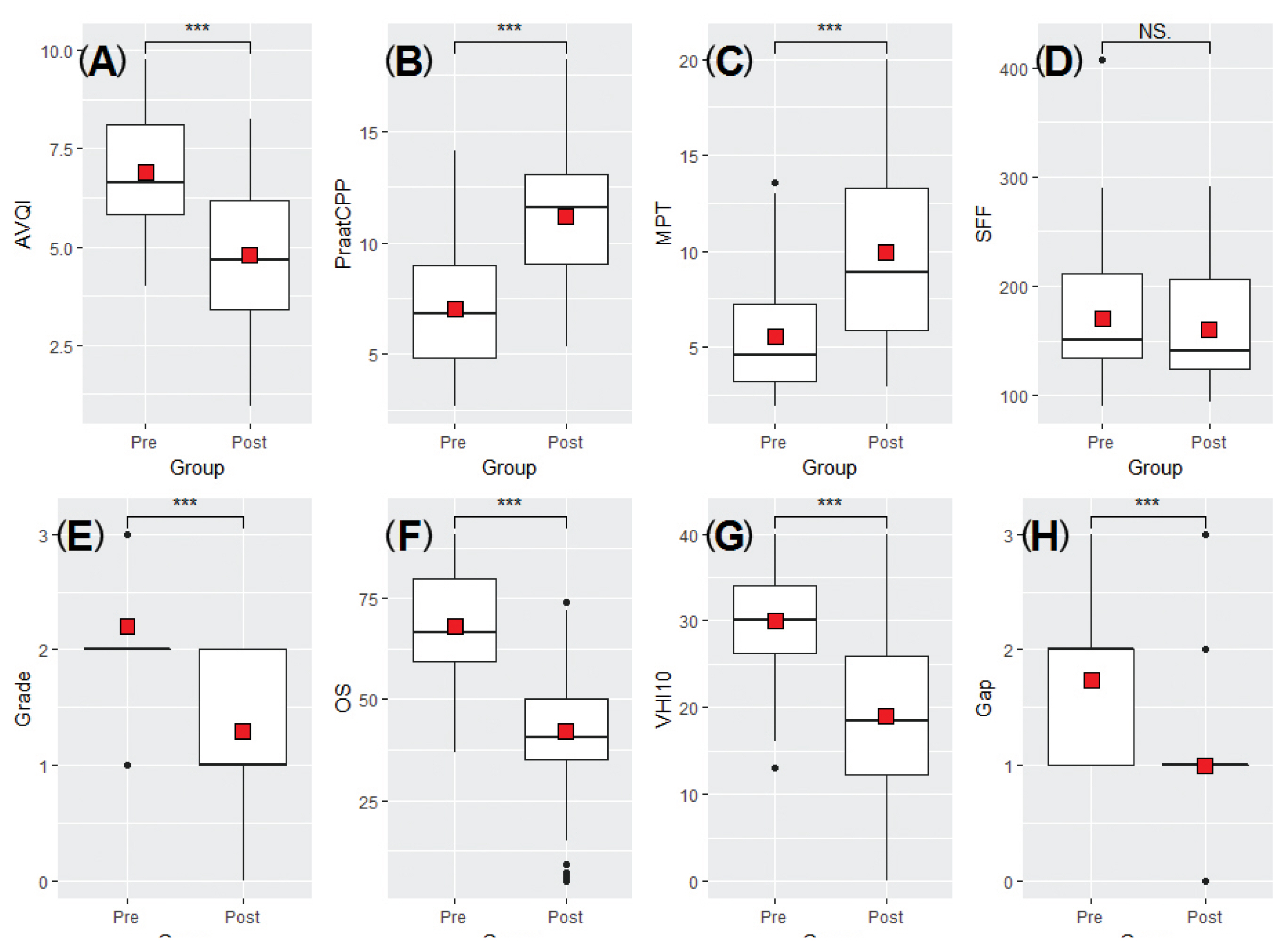
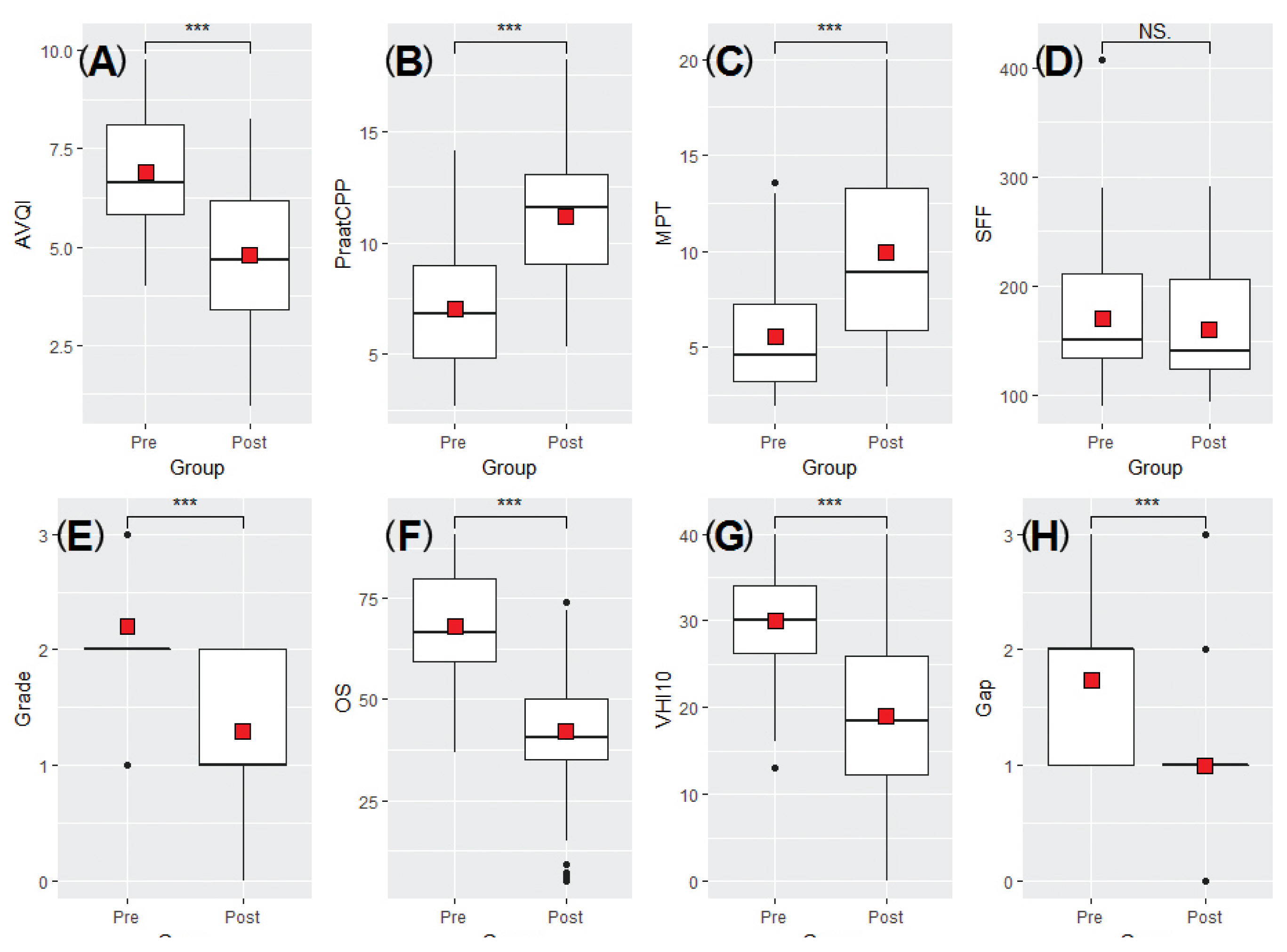
- Figure 1 and Table 2 show the mean values measured before and after IL for all variables. As shown in Table 2, statistically significant changes were observed in all variables except SFF, before and after IL. The post-IL values of AVQI, SFF, Grade, OS, Gap, and VHI-10 were lower than those before IL; however, CPPS and MPT values increased. Based on this, it was confirmed that vocal fold function, including voice quality, was improved after treatment with IL.
- 3. Comparison of all variables pre- and post-IL, according to gender
- As shown in Table 3, AVQI, CPPS, MPT, Grade, OS, Gap, and VHI-10 showed significant improvements when comparing pre- and post-IL values in both the male and female groups. After IL treatment, SFF had decreased, though it was not significant.
- 4. Correlation among all variables before and after IL
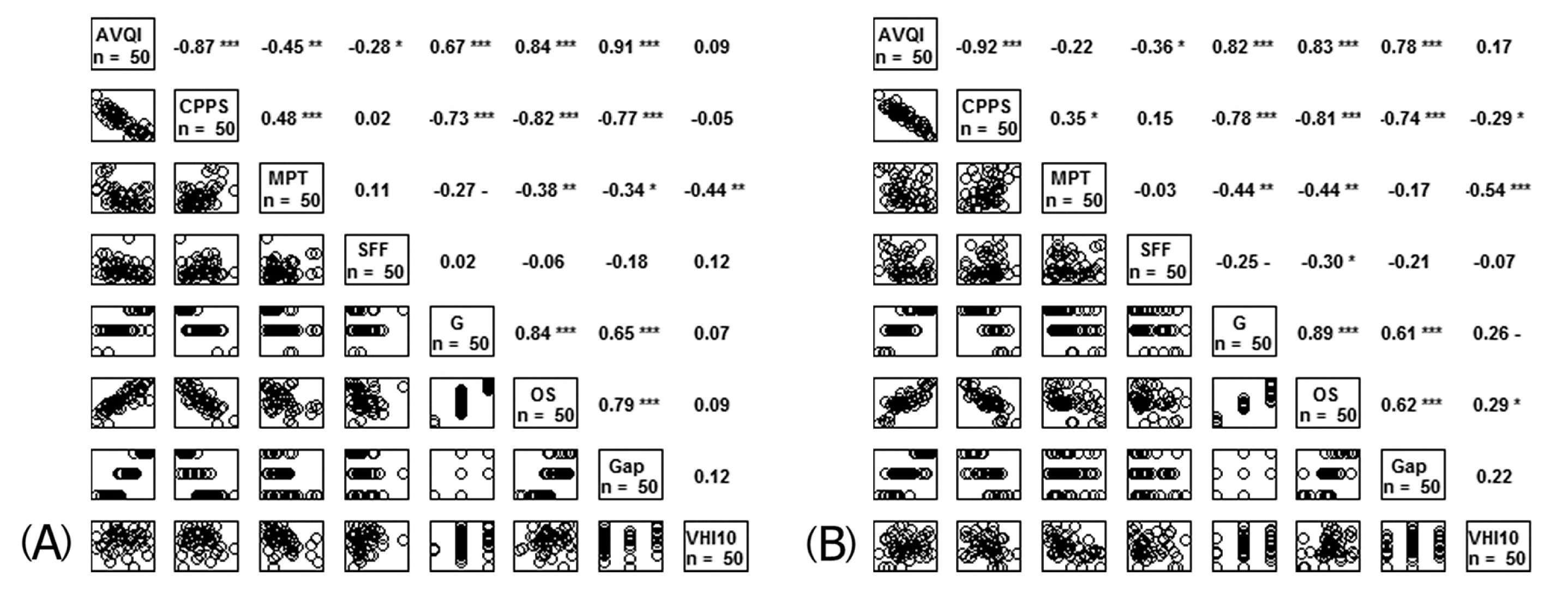
- The correlations amongst all variables before and after IL are shown in Figure 2. Prior to IL, AVQI had a significant correlation with CPPS (r = −0.87, p < 0.01), MPT (r = −0.45, p < 0.01), Grade (r = 0.67, p < 0.01), OS (r = 0.84, p < 0.01), and Gap (r = 0.91, p < 0.01). CPPS correlated with MPT (r = 0.48, p < 0.01), Grade (r = −0.73, p < 0.01), OS (r = −0.82, p < 0.01), and Gap (r = −0.77, p < 0.01). MPT had a significant correlation with Grade (r = −0.27, p < 0.01), OS (r = −0.38, p < 0.01), and Gap (r = −0.34, p < 0.05). Grade was correlated with OS (r = 0.84, p < 0.01) and Gap (r = 0.65, p < 0.01). A significant correlation was observed between OS and Gap (r = 0.79, p < 0.01). SFF and VHI-10 were not correlated with other variables in terms of pre-IL measurements.
- After treatment with IL, AVQI had a significant correlation with CPPS (r = −0.92, p < 0.01), Grade (r = 0.82, p < 0.01), OS (r = 0.83, p < 0.01), and Gap (r = 0.78, p < 0.01). CPPS was correlated with MPT (r = 0.35, p < 0.05), Grade (r = −0.78, p < 0.01), OS (r = −0.81, p < 0.01), and Gap (r = −0.74, p < 0.01). MPT had a significant correlation with Grade (r = −0.44, p < 0.01), OS (r = −0.44, p < 0.01), and VHI-10 (r = −0.54, p < 0.01). Grade was correlated with OS (r = 0.89, p < 0.01) and Gap (r = 0.61, p < 0.01). Significant correlation was found between OS and Gap (r = 0.62, p < 0.01). SFF showed no correlation with other variables.
Results
- The voice quality of the patients with VFP before and after IL treatment were compared by performing acoustic analysis (AVQI, CPPS, MPT, SFF), auditory-perceptual evaluation (Grade and OS), visual judgment assessment (Gap) and by administering the VHI-10 self-questionnaire. All analyses showed significant positive changes and predicted voice recovery. In addition, the acoustic parameters showed a significant correlation between the auditory evaluation and visual judgment, but there was no correlation with the self-questionnaire. This indicated that the quantification of voice recovery, and the degree of recovery felt by the patient were different. Nonetheless, the self-questionnaire is important in that it reflects patient satisfaction with the level of voice recovery [27].
- IL had significant effects on AVQI, CPPS, MPT, Grade, OS, Gap, and VHI-10, but not on SFF. The benefit of IL was evident, as it significantly decreased the measurements of AVQI, Grade, OS, Gap, and VHI-10 and increased CPPS and MPT. These changes could be attributed to the decrease in glottal gap, following IL. In addition, the scores for Grade, OS, and VHI-10 decreased because the patients’ voices had improved according to the speech-language pathologists, and the patients themselves. Although the glottal gaps were approximated after IL, there was no change in SFF. The mean SFF after IL was reduced by about 15 Hz in men, although not to a significant level (p = 0.159). IL led to a positive change in the SFF, but this was not significant. This result can be interpreted based on the fact that the mean SFF before IL, did not deviate much from the normal SFF range in men. In women, there was no significant difference between the pre- and post-IL results (p = 0.853), which suggests that the mean SFF before IL, was within the normal SFF range. These results are consistent with those of a previous study [28]. In other studies, the post-IL patient groups tended to have lower normalized scores, and significant improvements were observed in GRBAS, MPT, CPPS, closed quotient (CQ), voice range profile, semi-tone range, and VHI-10 scores [29,30].
- Most studies used the Multi-Dimensional Voice Program (MDVP, perturbation analysis method) to evaluate vocal fold recovery following surgical and behavioral interventions in patients with voice disorders [31,32]. However, if a very rough or breathy voice is analyzed by MDVP, it is unable to detect the voice cycle peak in the voice signal, and thus cannot produce accurate results. Despite the ability of MDVP to assess the pathological voice, traditional perturbation analyses performed in previous studies were shown to be untrustworthy components of the disordered voice [33]. The reason for this unpredictability is that the disordered voice may not be logically correlated with a single perturbation analysis. Another possibility is that because these measurements depend on the ability to detect periodicity of the voice (determining fundamental frequency), small errors in tracking periodicity can result in significant errors when using the perturbation method. Thus, because of the difficulty in detecting periodicity, obtaining precise quantifications of periodicity in pathological voice samples that include diplophonia or a severe grade of breathy and rough voice, may be challenging. CPP and CPPS were introduced to solve this limitation [34]. CPP and CPPS values are affected by pause and unvoiced interval duration in the voice tasks; this leads to fluctuations in these measurements, depending on whether the testing includes the sustained vowel and connected speech tasks.
- To our knowledge, this study is the first to use AVQI measurements to determine the effect of IL on VFP patients. AVQI measurements quantify the dysphonia severity by assessing a combination of several variables, rather than just 1 acoustical index. AVQI has made it possible to analyze the samples that concatenate sustained vowel phonations and connected speech [9]. Previous studies have confirmed a high correlation between the CPPS and the AVQI giving them clinical utility [35]. In this study, multidimensional evaluation methods were used to evaluate voice improvement after IL. Of the variables analyzed in this study, AVQI was the least commonly used, and it was designed to be automatically analyzed using the Praat script [36]. The Praat script was modified to suit the purpose of this study. AVQI was calculated using the Praat script, so that anyone who understood the script could easily operate it. The severity of pathological voice was quantified through the voiced speech segments extracted from the same speech sample content (2 seconds of vowel sound + 25 syllables), so an objective comparison of the degree of speech impairment was possible. Thus, the AVQI confirmed the effect of IL, similar to that determined using other variables. In previous studies, patients with VFP showed higher AVQI, Grade, and OS values when compared with those of the control group [37]. CPPS showed a high correlation with the perception of breathiness [34]. Normal participants have larger CPPS values than those of dysphonic patients. Based on this characteristic, if the CPPS value increased after IL, improvement of the voice can be expected. These results were consistent with a previous study, in which a larger phonatory gap led to lower values of CPPS [38].
- IL continues to evolve as new techniques, approaches, and injectable materials are developed. Recently, HA was introduced as an ideal injectable material, and it has since been widely used in clinical practice [39]. HA absorbs the impact that occurs when the vocal fold vibrates, and it plays an important role in maintaining the shape and characteristic viscoelasticity of the vocal fold [40]. Due to heterologous species having the same HA chemical structure, there is no basis for rejection, making xenotransplantation possible [6]. It is also introduced as a material suitable for temporary vocal fold adduction because it decomposes spontaneously in vivo. Additionally, it is considered to be an ideal injectable material for temporary vocal fold adduction because it remains in vivo for 6–12 months and maintains function for 3–4 months [7]. Considering the possibility of natural recovery from VFP, it is appropriate to select a temporary injectable material, such as HA. Previous studies have reported on voice recovery using traditional acoustic analyses, such as perturbation analysis, following HA injection, and this study also confirmed voice recovery by AVQI after IL [41,42].
- Many studies have evaluated the effect of other injectable substances on voice recovery. Some patients underwent endoscopic injection with polydimethylsiloxane for the treatment of unilateral vocal fold paralysis (UVFP). After the polydimethylsiloxane injection, all acoustic (MPT), auditory-perceptual (grade, instability, roughness, breathiness, asthenia, and strain; GIRBAS), visual judgment (vocal fold position and glottal closure), and VHI subjective assessments showed significant improvement [43]. Cases of injection with calcium hydroxylapatite and autologous fascia in patients with UVFP have been reported [44,45]. Injection with calcium hydroxylapatite improved voice quality (laryngoscopic findings and CAPE-V scores) in both irradiated and non-irradiated patients. HA degrades in vivo, does not cause an inflammatory reaction, and has excellent viscoelasticity. It is mainly used to temporarily alleviate symptoms, such as breathiness and aspiration, in unilateral VFP patients who are likely to recover [46]. Non-absorbable synthetic materials such as calcium hydroxyapatite gel, polydimethylsiloxane gel, and Teflon have been introduced for the correction of permanent VFP. However, if the possibility of recovery is uncertain, as in the case of this study, it may be disadvantageous to use non-absorbable synthetic materials [47]. Additionally, it was reported that IL using autologous fascia appeared to be a reliable and promising method to help in the recovery of voice quality. However, this study was limited to patients who underwent HA injection; hence, further studies are needed to confirm voice changes through AVQI with respect to using other injection materials. These efforts will be of great help to otolaryngologists, speech language pathologists, and patients with voice disorders.
- The results of this study are expected to provide a voice quantification criterion that predicts voice recovery. In particular, regarding VFP patients, analysis of concatenated sustained vowel phonations and connected speech, provide more accurate and diverse information than analysis of a single voice task. Voice evaluation through various tasks can provide greater evidence of voice recovery. It is possible to assess the pathological voice through various tasks. A comprehensive evaluation of voice quality makes it possible to more accurately predict prognosis following intervention. Therefore, these evaluation procedures should be continually developed.
- In conclusion, the results of the multidimensional assessment of vocal fold function in patients with VFP indicated that voice recovery was achieved after IL. Although significant changes were not observed in the SFF before and after treatment, the values of the other measured variables including AVQI, CPPS, MPT, Grade, OS, Gap, and VHI-10 were improved. In addition, because measured values varied according to the vowel duration, and voiced segments (sentence contents) used in the voice evaluation, it is important that an appropriate voice sample representing the characteristics of the dysphonia be selected for the multidimensional assessment.
Discussion
- 1. McCulloch TM, Andrews BT, Hoffman HT, Graham SM, Karnell MP, Minnick C. Long-term follow-up of fat injection laryngoplasty for unilateral vocal cord paralysis. Laryngoscope 2002;112(7). 1235−8. PMID: 10.1097/00005537-200207000-00017. PMID: 12169905.ArticlePubMed
- 2. Friedman AD, Burns JA, Heaton JT, Zeitels SM. Early Versus Late Injection Medialization for Unilateral Vocal Cord Paralysis. Laryngoscope 2010;120(10). 2042−6. PMID: 10.1002/lary.21097. PMID: 20824787.ArticlePubMed
- 3. Rosenthal LH, Benninger MS, Deeb RH. Vocal fold immobility: a longitudinal analysis of etiology over 20 years. Laryngoscope 2007;117(10). 1864−70. PMID: 10.1097/MLG.0b013e3180de4d49. PMID: 17713451.ArticlePubMed
- 4. Morgan JE, Zraick RI, Griffin AW, Bowen TL, Johnson FL. Injection versus medialization laryngoplasty for the treatment of unilateral vocal fold paralysis. Laryngoscope 2007;117(11). 2068−74. PMID: 10.1097/MLG.0b013e318137385e. PMID: 17828043.ArticlePubMed
- 5. Cha W, Ro JH, Wang SG, et al. Development of a device for real-time light-guided vocal fold injection: A preliminary report. Laryngoscope 2016;126(4). 936−40. PMID: 10.1002/lary.25661.ArticlePubMed
- 6. Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg 2004;20(1). 21−9. PMID: 10.1055/s-2004-822955. PMID: 15034810.ArticlePubMedPDF
- 7. Kwon TK, Buckmire R. Injection laryngoplasty for management of unilateral vocal fold paralysis. Curr Opin Otolaryngol Head Neck Surg 2004;12(6). 538−42. PMID: 10.1097/01.moo.0000144393.40874.98. PMID: 15548914.ArticlePubMed
- 8. Ma EPM, Yiu EML. Voice activity and participation profile: Assessing the impact of voice disorders on daily activities. J Speech Lang Hear Res 2001;44(3). 511−24. PMID: 10.1044/1092-4388(2001/040). PMID: 11407557.ArticlePubMed
- 9. Maryn Y, De Bodt M, Roy N. The Acoustic Voice Quality Index: Toward improved treatment outcomes assessment in voice disorders. J Commun Disord 2010;43(3). 161−74. PMID: 10.1016/j.jcomdis.2009.12.004. PMID: 20080243.ArticlePubMed
- 10. Tan ML, Woo P. Injection Laryngoplasty with Micronized Dermis: A 10-Year Experience with 381 Injections in 344 Patients. Laryngoscope 2010;120(12). 2460−6. PMID: 10.1002/lary.21130. PMID: 20972969.ArticlePubMed
- 11. Lee SW, Son YI, Kim CH, Lee JY, Kim SC, Koh YW. Voice outcomes of polyacrylamide hydrogel injection laryngoplasty. Laryngoscope 2007;117(10). 1871−5. PMID: 10.1097/MLG.0b013e3180caa1b1. PMID: 17690620.ArticlePubMed
- 12. Wong BY-H, Yu S-Y, Ho W-K, Wei WI, Ng ML. Injection laryngoplasty using hyaluronic acid for Chinese patients with unilateral vocal fold paralysis. Speech Lang Hear 2016;19(3). 153−60. PMID: 10.1080/2050571X.2016.1159407.Article
- 13. Ng M, Wong B. Voice performance in tonal language speakers with glottal insufficiency due to unilateral vocal fold paralysis after injection laryngoplasty: a multidimensional study of Cantonese patients. Hong Kong Med J 2018;24:Suppl 2. (1). 42−4. PMID: 29938658.
- 14. De Bodt MS, Wuyts FL, Van de Heyning PH, Croux C. Test-retest study of the GRBAS scale: Influence of experience and professional background on perceptual rating of voice quality. J Voice 1997;11(1). 74−80. PMID: 10.1016/S0892-1997(97)80026-4. PMID: 9075179.ArticlePubMed
- 15. Lee JM, Roy N, Peterson E, Merrill RM. Comparison of Two Multiparameter Acoustic Indices of Dysphonia Severity: The Acoustic Voice Quality Index and Cepstral Spectral Index of Dysphonia. J Voice 2018;32(4). 515.e1−13. PMID: 10.1016/j.jvoice.2017.06.012.ArticlePubMed
- 16. Uloza V, Petrauskas T, Padervinskis E, Ulozaite N, Barsties B, Maryn Y. Validation of the Acoustic Voice Quality Index in the Lithuanian Language. J Voice 2017;31(2). 257.e1−11. PMID: 10.1016/j.jvoice.2016.06.002.ArticlePubMed
- 17. Nemr K, Simoes-Zenari M, Cordeiro GF, et al. GRBAS and Cape-V Scales: High Reliability and Consensus When Applied at Different Times. J Voice 2012;26(6). 812.e17−22. PMID: 10.1016/j.jvoice.2012.03.005.ArticlePubMed
- 18. De Virgilio A, Chang M-H, Jiang R-S, et al. Influence of superior laryngeal nerve injury on glottal configuration/function of thyroidectomy-induced unilateral vocal fold paralysis. Otolaryngol Head Neck Surg 2014;151(6). 996−1002. PMID: 10.1177/0194599814549740. PMID: 25214548.ArticlePubMed
- 19. Vaca M, Cobeta I, Mora E, Reyes P. Clinical Assessment of Glottal Insufficiency in Age-related Dysphonia. J Voice 2017;31(1). 128.e1−e5. PMID: 10.1016/j.jvoice.2015.12.010.ArticlePubMed
- 20. Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the voice handicap index-10. Laryngoscope 2004;114(9). 1549−56. PMID: 10.1097/00005537-200409000-00009. PMID: 15475780.ArticlePubMed
- 21. Maryn Y, Kim HT, Kim J. Auditory-Perceptual and Acoustic Methods in Measuring Dysphonia Severity of Korean Speech. J Voice 2016;30(5). 587−94. PMID: 10.1016/j.jvoice.2015.06.011.ArticlePubMed
- 22. Hillenbrand J, Houde RA. Acoustic correlates of breathy vocal quality: dysphonic voices and continuous speech. J Speech Lang Hear Res 1996;39(2). 311−21. PMID: 10.1044/jshr.3902.311.Article
- 23. Maslan J, Leng X, Rees C, Blalock D, Butler SG. Maximum phonation time in healthy older adults. J Voice 2011;25(6). 709−13. PMID: 10.1016/j.jvoice.2010.10.002. PMID: 21439778. PMID: 3128209.ArticlePubMedPMC
- 24. Gelfer MP, Denor SL. Speaking Fundamental Frequency and Individual Variability in Caucasian and African American School-Age Children. Am J Speech-Lang Pat 2014;23(3). 395−406. PMID: 10.1044/2014_AJSLP-13-0016.Article
- 25. Hirano M. Psyco-acoustic evaluation of voice. Clinical Examination of Voice. Springer-Verlag; 1981. pp 81−4.
- 26. Karnell MP, Melton SD, Childes JM, Coleman TC, Dailey SA, Hoffman HT. Reliability of Clinician-Based (GRBAS and CAPE-V) and Patient-Based (V-RQOL and IPVI) Documentation of Voice Disorders. J Voice 2007;21(5). 576−90. PMID: 10.1016/j.jvoice.2006.05.001.ArticlePubMed
- 27. Pinarbasli MO, Kaya E, Ozudogru E, et al. Acoustic Analysis of Soccer Fans in Acute Phonotrauma After the Match. J Voice [Preprint] 2017 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0892-1997(17)30294-1. PMID: 10.1016/j.jvoice.2017.10.004.Article
- 28. Bae SH, Lee SJ. Comparison of vocal outcome after autologous fat injection and medialization thyroplasty for unilateral vocal cord paralysis. Korean J Otorhinolaryngol-Head Neck Surg 2010;53(1). 24−9. PMID: 10.3342/kjorl-hns.2010.53.1.24.Article
- 29. Hartl DM, Hans S, Vaissière J, Riquet M, Brasnu DF. Objective Voice Quality Analysis Before and After Onset of Unilateral Vocal Fold Paralysis. J Voice 2001;15(3). 351−61. PMID: 10.1016/S0892-1997(01)00037-6. PMID: 11575632.ArticlePubMed
- 30. Mattioli F, Bettini M, Botti C, et al. Polydimethylsiloxane Injection Laryngoplasty for Unilateral Vocal Fold Paralysis: Long-Term Results. J Voice 2017;31(4). 517.e1−7. PMID: 10.1016/j.jvoice.2016.12.017.ArticlePubMed
- 31. Vaz-Freitas S, Pestana PM, Almeida V, Ferreira A. Acoustic analysis of voice signal: Comparison of four applications software. Biomed Signal Process Control 2018;40:318−23. PMID: 10.1016/j.bspc.2017.09.031.Article
- 32. Putzer M. Multiparametric description of voice quality for normal male and female voices. Folia Phoniatr Logop 2001;53(2). 73−84. [in German]. PMID: 11244281.ArticlePubMed
- 33. Wolfe V, Fitch J, Cornell R. Acoustic prediction of severity in commonly occurring voice problems. J Speech Lang Hear Res 1995;38(2). 273−9. PMID: 10.1044/jshr.3802.273.Article
- 34. Hillenbrand J, Cleveland RA, Erickson RL. Acoustic Correlates of Breathy Vocal Quality. J Speech Lang Hear Res 1994;37:769−78. PMID: 10.1044/jshr.3704.769.Article
- 35. Maryn Y, Weenink D. Objective Dysphonia Measures in the Program Praat: Smoothed Cepstral Peak Prominence and Acoustic Voice Quality Index. J Voice 2015;29(1). 35−43. PMID: 10.1016/j.jvoice.2014.06.015.ArticlePubMed
- 36. Maryn Y, De Bodt M, Barsties B, Roy N. The value of the Acoustic Voice Quality Index as a measure of dysphonia severity in subjects speaking different languages. Eur Arch Otorhinolaryngol 2014;271(6). 1609−19.ArticlePubMedPDF
- 37. Kim G-H, Lee Y-W, Park H-J, Bae I-H, Lee B-J, Kwon S-B. Auditory-Perceptual and Acoustic Evaluation in Measuring Dysphonia Severity of Vocal Cord Paralysis. J Korean Soc Laryngol Phoniatr Logop 2017;28(2). 106−11. PMID: 10.22469/jkslp.2017.28.2.106.ArticlePDF
- 38. Balasubramanium RK, Bhat JS, Fahim S, Raju R. Cepstral Analysis of Voice in Unilateral Adductor Vocal Fold Palsy. J Voice 2011;25(3). 326−9. PMID: 10.1016/j.jvoice.2009.12.010.ArticlePubMed
- 39. Courey MS. Injection laryngoplasty. Otolaryngol Clin North Am 2004;37(1). 121−38. PMID: 10.1016/j.otc.2003.12.002. PMID: 15062690.ArticlePubMed
- 40. Gaston J, Thibeault SL. Hyaluronic acid hydrogels for vocal fold wound healing. Biomatter 2013;3(1). 1−7. PMID: 10.4161/biom.23799.Article
- 41. Reiter R, Brosch S. Laryngoplasty with hyaluronic acid in patients with unilateral vocal fold paralysis. J Voice 2012;26(6). 785−91. PMID: 10.1016/j.jvoice.2011.11.007. PMID: 22578435.ArticlePubMed
- 42. Branco A, Rodrigues SA, Fabro AT, Fonseca-Alves CE, Martins RH. Hyaluronic acid behavior in the lamina propria of the larynx with advancing age. Otolaryngol Head Neck Surg 2014;151(4). 652−6. PMID: 10.1177/0194599814544673. PMID: 25096358.ArticlePubMed
- 43. Bergamini G, Alicandri-Ciufelli M, Molteni G, et al. Therapy of Unilateral Vocal Fold Paralysis With Polydimethylsiloxane Injection Laryngoplasty: Our Experience. J Voice 2010;24(1). 119−25. PMID: 10.1016/j.jvoice.2008.05.003.ArticlePubMed
- 44. Chang J, Courey MS, Al-Jurf SA, Schneider SL, Yung KC. Injection Laryngoplasty Outcomes in Irradiated and Nonirradiated Unilateral Vocal Fold Paralysis. Laryngoscope 2014;124(8). 1895−9. PMID: 10.1002/lary.24622. PMID: 24473831.ArticlePubMed
- 45. Rihkanen H, Lehikoinen-Soderlund S, Reijonen P. Voice acoustics after autologous fascia injection for vocal fold paralysis. Laryngoscope 1999;109(11). 1854−8. PMID: 10.1097/00005537-199911000-00026. PMID: 10569422.ArticlePubMed
- 46. Chan RW, Titze IR. Hyaluronic acid (with fibronectin) as a bioimplant for the vocal fold mucosa. Laryngoscope 1999;109(7). 1142−9. PMID: 10.1097/00005537-199907000-00026. PMID: 10401858.ArticlePubMed
- 47. Chan RW, Gray SD, Titze IR. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngology—Head and Neck Surgery 2001;124(6). 607−14. PMID: 10.1177/019459980112400602.Article
References
| Variables | Pre-injection | Post-injection | t | p |
|---|---|---|---|---|
| AVQI | 6.91 ± 1.57 | 4.7 9± 1.88 | 6.75 | < 0.001** |
|
|
||||
| CPPS | 7.02 ± 2.66 | 11.20 ± 2.95 | −7.77 | < 0.001** |
|
|
||||
| MPT | 5.52 ± 2.89 | 9.94 ± 5.01 | −7.27 | < 0.001** |
|
|
||||
| SFF | 170.70 ± 60.27 | 159.85 ± 5 1.34 | 1.51 | 0.138 |
|
|
||||
| Grade | 2.22 ± 0.49 | 1.3 ± 0.65 | 8.65 | < 0.001** |
|
|
||||
| OS | 68.08 ± 12.94 | 42.04 ± 15.87 | 10.48 | < 0.001** |
|
|
||||
| Mean Gap | 1.74 ± 0.80 | 0.98 ± 0.68 | 5.72 | < 0.001** |
| - Median | 0 | 12 | ||
| - Paramedian | 24 | 27 | ||
| - Intermediate | 15 | 11 | ||
| - Fully abducted | 11 | 0 | ||
|
|
||||
| VHI-10 | 30.00 ± 6.38 | 19.06 ± 9.53 | 9.13 | < 0.001** |
| Variables | Male | Female | ||||
|---|---|---|---|---|---|---|
| Pre-injection | Post-injection | p | Pre-injection | Post-injection | p | |
| AVQI | 7.2 ± 1.6 | 5.2 ± 1.9 | < 0.001** | 6.4 ± 1.5 | 4.0 ± 1.6 | < 0.001** |
|
|
||||||
| CPPS | 6.9 ± 2.9 | 11.0 ± 3.3 | < 0.001** | 7.3 ± 2.1 | 11.7 ± 2.2 | < 0.001** |
|
|
||||||
| MPT | 5.3 ± 2.8 | 10.2 ± 5.4 | < 0.001** | 6.0 ± 3.1 | 9.4 ± 4.2 | 0.011* |
|
|
||||||
| SFF | 146.9 ± 54.0 | 131.9 ± 26.6 | 0.159 | 216.9 ± 43.0 | 214.2 ± 43.6 | 0.853 |
|
|
||||||
| Grade | 2.24 ± 0.56 | 1.42 ± 0.61 | < 0.001** | 2.11 ± 0.33 | 1.05 ± 0.66 | < 0.001** |
|
|
||||||
| OS | 68.8 ± 13.4 | 44.8 ± 14.8 | < 0.001** | 66.5 ± 12.2 | 36.6 ± 17.0 | < 0.001** |
|
|
||||||
| Mean Gap | 1.82 ± 0.85 | 1.03 ± 0.73 | 0.001** | 1.58 ± 0.71 | 0.88 ± 0.60 | 0.042* |
|
|
||||||
| - Median | 0 | 8 | 0 | 4 | ||
|
|
||||||
| - Paramedian | 15 | 16 | 9 | 11 | ||
|
|
||||||
| - Intermediate | 9 | 9 | 6 | 2 | ||
|
|
||||||
| - Fully abducted | 9 | 0 | 2 | 0 | ||
|
|
||||||
| VHI-10 | 30.0 ± 6.5 | 19.5 ± 9.2 | < 0.001** | 30.1 ± 6.3 | 18.2 ± 10.4 | < 0.001** |
Figure & Data
References
Citations

- The Long Term Results of Hyaluronic Acid/Dextranomer Injection Laryngoplasty in Unilateral Vocal Fold Paralysis
Elvin Alaskarov, Ayşegül Batıoğlu-Karaaltın, Zülküf Burak Erdur, Züleyha Dilek Gülmez, Hakkı Caner İnan, Özcan Öztürk
Annals of Otology, Rhinology & Laryngology.2024;[Epub] CrossRef - Primary Nonselective Laryngeal Reinnervation in Iatrogenic Acute Recurrent Laryngeal Nerve Injury: Case Series and Literature Review
Wan Nabila Wan Mansor, Mawaddah Azman, Rabani Remli, Mohd Razif Mohamad Yunus, Marina Mat Baki
Ear, Nose & Throat Journal.2023; 102(3): 164. CrossRef - Accuracy of Acoustic Voice Quality Index Captured With a Smartphone – Measurements With Added Ambient Noise
Virgilijus Uloza, Nora Ulozaitė-Stanienė, Tadas Petrauskas, Rima Kregždytė
Journal of Voice.2023; 37(3): 465.e19. CrossRef - Efficacy of Injectable Laryngoplasty With Hyaluronic Acid and/or Calcium Hydroxyapatite in the Treatment of Glottic Incompetence. Systematic Review and Meta-analysis
Débora Pereira Henriques, Regina Helena Garcia Martins, Antônio José Maria Cataneo
Journal of Voice.2023;[Epub] CrossRef - Rekonstruktionsmöglichkeiten des Larynx
M. Goncalves, J. Taeger, S. Hackenberg
Die MKG-Chirurgie.2023; 16(2): 138. CrossRef - Injection laryngoplasty during transoral laser microsurgery for early glottic cancer: a randomized controlled trial
Ayham Al Afif, Matthew H. Rigby, Colin MacKay, Timothy F. Brown, Timothy J. Phillips, Usman Khan, Jonathan R. B. Trites, Martin Corsten, S. Mark Taylor
Journal of Otolaryngology - Head & Neck Surgery.2022;[Epub] CrossRef - Meta-Analysis on the Validity of the Acoustic Voice Quality Index
Christina Batthyany, Ben Barsties V. Latoszek, Youri Maryn
Journal of Voice.2022;[Epub] CrossRef - Injection laryngoplasty with hyaluronic acid for glottal insufficiency in unilateral vocal fold paralysis: a systematic review of the literature
A. Švejdová, J. Dršata, J. Mejzlík, M. Homoláč, J. Krtičková, J. Šatanková, V. Chrobok
European Archives of Oto-Rhino-Laryngology.2022; 279(11): 5071. CrossRef - Can preoperative results predict the need for future reintervention following injection laryngoplasty for unilateral vocal fold paralysis?
Beata Miaśkiewicz, Aleksandra Panasiewicz, Elżbieta Gos, Paulina Krasnodębska, Piotr H. Skarżyński, Agata Szkiełkowska
European Archives of Oto-Rhino-Laryngology.2021; 278(10): 3883. CrossRef


 PubReader
PubReader Cite
Cite