Search
- Page Path
- HOME > Search
Special Article
- The COVID-19 Vaccine Safety Research Center: a cornerstone for strengthening safety evidence for COVID-19 vaccination in the Republic of Korea
- Na-Young Jeong, Hyesook Park, Sanghoon Oh, Seung Eun Jung, Dong-Hyun Kim, Hyoung-Shik Shin, Hee Chul Han, Jong-Koo Lee, Jun Hee Woo, Jaehun Jung, Joongyub Lee, Ju-Young Shin, Sun-Young Jung, Byung-Joo Park, Nam-Kyong Choi
- Received November 16, 2023 Accepted February 22, 2024 Published online April 4, 2024
- DOI: https://doi.org/10.24171/j.phrp.2023.0343 [Epub ahead of print]
- 340 View
- 22 Download
-
 Abstract
Abstract
 PDF
PDF - The COVID-19 Vaccine Safety Research Committee (CoVaSC) was established in November 2021 to address the growing need for independent, in-depth scientific evidence on adverse events (AEs) following coronavirus disease 2019 (COVID-19) vaccination. This initiative was requested by the Korea Disease Control and Prevention Agency and led by the National Academy of Medicine of Korea. In September 2022, the COVID-19 Vaccine Safety Research Center was established, strengthening CoVaSC’s initiatives. The center has conducted various studies on the safety of COVID-19 vaccines. During CoVaSC’s second research year, from September 29, 2022 to July 19, 2023, the center was restructured into 4 departments: Epidemiological Research, Clinical Research, Communication & Education, and International Cooperation & Policy Research. Its main activities include (1) managing CoVaSC and the COVID-19 Vaccine Safety Research Center, (2) surveying domestic and international trends in AE causality investigation, (3) assessing AEs following COVID-19 vaccination, (4) fostering international collaboration and policy research, and (5) organizing regular fora and training sessions for the public and clinicians. Causality assessments have been conducted for 27 diseases, and independent research has been conducted after organizing ad hoc committees comprising both epidemiologists and clinical experts on each AE of interest. The research process included protocol development, data analysis, interpretation of results, and causality assessment. These research outcomes have been shared transparently with the public and healthcare experts through various fora. The COVID-19 Vaccine Safety Research Center plans to continue strengthening and expanding its research activities to provide reliable, high-quality safety information to the public.
Review Article
- Psychiatric adverse events associated with the COVID-19 vaccines approved in the Republic of Korea: a systematic review
- Seungeun Ryoo, Miyoung Choi, Nam-Kyong Choi, Hyoung-Shik Shin, Jun Hee Woo, Byung-Joo Park, Sanghoon Oh
- Received October 31, 2023 Accepted January 16, 2024 Published online March 28, 2024
- DOI: https://doi.org/10.24171/j.phrp.2023.0325 [Epub ahead of print]
- 413 View
- 27 Download
-
 Abstract
Abstract
 PDF
PDF - This systematic review evaluated psychiatric adverse events (AEs) following vaccination against coronavirus disease 2019 (COVID-19). We included studies that reported or investigated psychiatric AEs in individuals who had received an approved COVID-19 vaccine in the Republic of Korea. Systematic electronic searches of Ovid-Medline, Embase, CENTRAL, and KoreaMed databases were conducted on March 22, 2023. Risk of bias was assessed using the Risk of Bias Assessment Tool for Non-randomized Studies 2.0. The study protocol was registered in the International Prospective Register of Systematic Reviews (CRD42023449422). Of the 301 articles initially selected, 7 were included in the final analysis. All studies reported on sleep disturbances, and 2 highlighted anxiety-related AEs. Sleep disorders like insomnia and narcolepsy were the most prevalent AEs, while depression was not reported. Our review suggests that these AEs may have been influenced by biological mechanisms as well as the broader psychosocial context of the COVID-19 pandemic. Although this study had limitations, such as a primary focus on the BNT162b2 vaccine and an observational study design, it offered a systematic, multi-vaccine analysis that fills a critical gap in the existing literature. This review underscores the need for continued surveillance of psychiatric AEs and guides future research to investigate underlying mechanisms, identify risk factors, and inform clinical management.
Original Article
- COVID-19 infection among people with disabilities in 2021 prior to the Omicron-dominant period in the Republic of Korea: a cross-sectional study
- Seul-Ki Kang, Bryan Inho Kim
- Received July 11, 2023 Accepted January 16, 2024 Published online March 28, 2024
- DOI: https://doi.org/10.24171/j.phrp.2023.0194 [Epub ahead of print]
- 371 View
- 8 Download
-
 Abstract
Abstract
 PDF
PDF - Objectives
This study investigated the characteristics of coronavirus disease 2019 (COVID-19) among individuals with disabilities on a nationwide scale in the Republic of Korea, as limited research has examined this population.
Methods
Between January 1 and November 30, 2021, a total of 5,687 confirmed COVID-19 cases among individuals with disabilities were reported through the Korea Disease Control and Prevention Agency’s COVID-19 web reporting system. Follow-up continued until December 24, and demographic, epidemiological, and clinical characteristics were analyzed.
Results
Individuals with disabilities represented approximately 1.5% of confirmed cases, with a mean age of 58.1 years. Most resided in or near metropolitan areas (86.6%) and were male (60.6%). Frequent sources of infection included home (33.4%) and contact with confirmed cases (40.7%). Many individuals (75.9%) had underlying conditions, and 7.7% of cases were severe. People with disabilities showed significantly elevated risk of severe infection (adjusted odds ratio [aOR], 1.63; 95% confidence interval [CI], 1.47–1.81) and mortality (aOR, 1.65; 95% CI, 1.43–1.91). Vaccination against COVID-19 was associated with significantly lower risk of severe infection (aORs for the first, second, and third doses: 0.6 [95% CI, 0.42–0.85], 0.28 [95% CI, 0.22–0.35], and 0.16 [95% CI, 0.05–0.51], respectively) and death (adjusted hazard ratios for the first and second doses: 0.57 [95% CI, 0.35–0.93] and 0.3 [95% CI, 0.23–0.40], respectively).
Conclusion
Individuals with disabilities showed higher risk of severe infection and mortality from COVID-19. Consequently, it is critical to strenghthenCOVID-19 vaccination initiatives and provide socioeconomic assistance for this vulnerable population.
Commentary
- Challenges in capacity building of national immunization programs and emergency or pandemic vaccination responses in the Global Health Security Agenda member countries
- Sookhyun Lee, Jung Ju Oh, Sang Hyun Park, Dasol Ro, Ye Jin Jeong, So Yoon Kim
- Received June 14, 2023 Accepted January 16, 2024 Published online March 28, 2024
- DOI: https://doi.org/10.24171/j.phrp.2023.0159 [Epub ahead of print]
- 196 View
- 9 Download
Original Article
- Evaluation of COVID-19 vaccine effectiveness in different high-risk facility types during a period of Delta variant dominance in the Republic of Korea: a cross-sectional study
- Min Jei Lee, Myung-Jae Hwang, Dong Seob Kim, Seon Kyeong Park, Jihyun Choi, Ji Joo Lee, Jong Mu Kim, Young-Man Kim, Young-Joon Park, Jin Gwack, Sang-Eun Lee
- Osong Public Health Res Perspect. 2023;14(5):418-426. Published online October 19, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0188
- 1,263 View
- 44 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 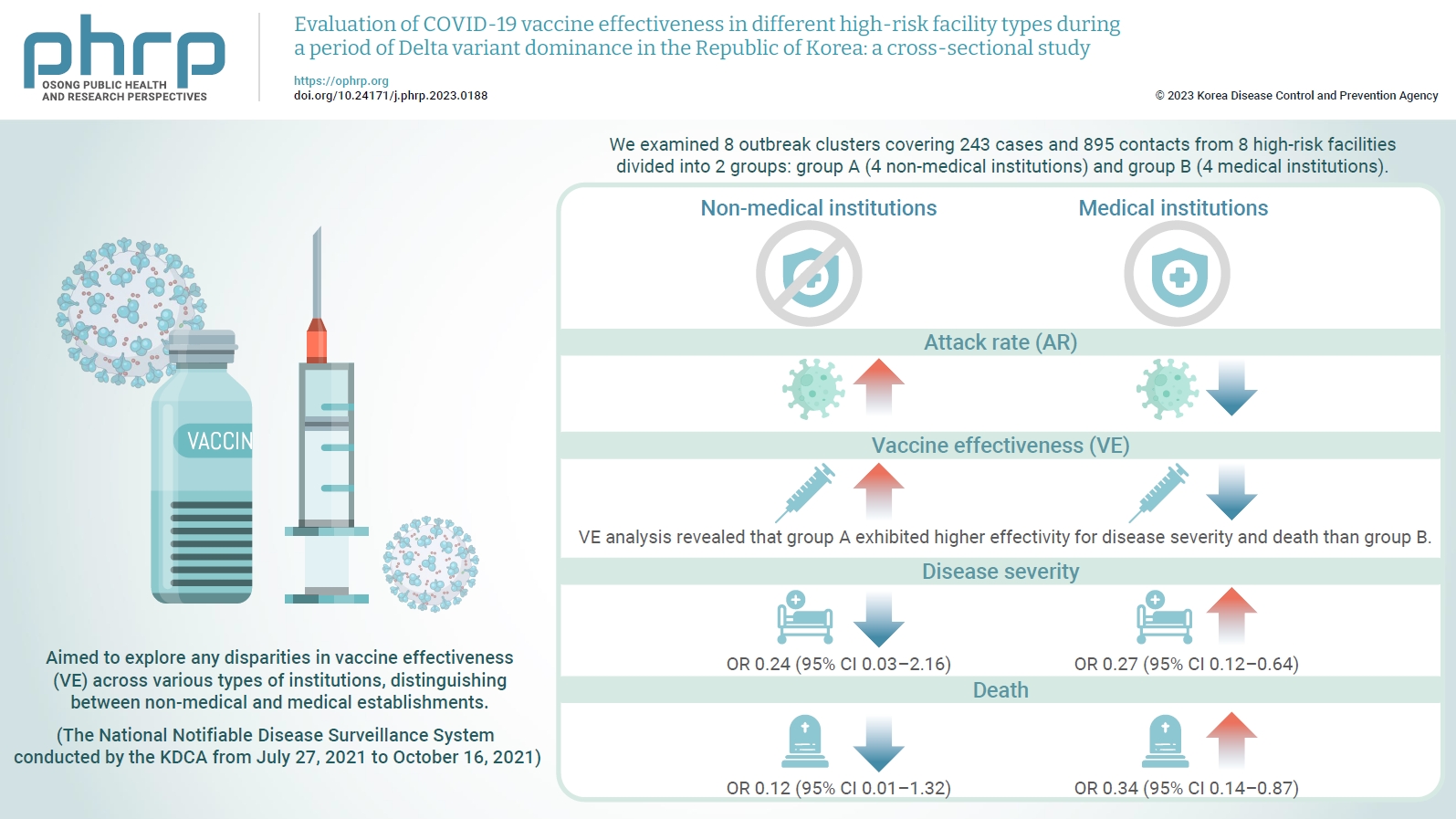
- Objectives
We evaluated the effectiveness of coronavirus disease 2019 vaccination in high-risk facilities in the Republic of Korea during the period when the highly transmissible Delta variant was prevalent. Additionally, we aimed to explore any disparities in vaccine effectiveness (VE) across various types of institutions, specifically distinguishing between non-medical and medical establishments. Methods: We examined 8 outbreak clusters covering 243 cases and 895 contacts from 8 high-risk facilities divided into 2 groups: group A (4 non-medical institutions) and group B (4 medical institutions). These clusters were observed from July 27, 2021 to October 16, 2021 for the attack rate (AR) and VE with respect to disease severity. A generalized linear model with a binomial distribution was used to determine the odds ratio (OR) for disease severity and death. Results: AR was notably lower in group B (medical institutions). Furthermore, VE analysis revealed that group A exhibited higher effectivity for disease severity and death than group B. The OR for disease severity was 0.24 (95% confidence interval [CI], 0.03–2.16) for group A and 0.27 (95% CI, 0.12–0.64) for group B, with the OR for death at 0.12 (95% CI, 0.01–1.32) in group A and 0.34 (95% CI, 0.14–0.87) in group B. Conclusion: Although VE may vary across institutions, our findings underscore the importance of implementing vaccinations in high-risk facilities. Customized vaccination programs, tailored response plans, and competent management personnel are essential for effectively addressing and mitigating public health challenges.
Brief Report
- JYNNEOS vaccine safety monitoring in the Republic of Korea, 2022: a cross-sectional study
- Jaeeun Lee, Seunghyun Lewis Kwon, Jinhee Park, Hyuna Bae, Hyerim Lee, Geun-Yong Kwon
- Osong Public Health Res Perspect. 2023;14(5):433-438. Published online October 18, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0182
- 877 View
- 38 Download
- 1 Web of Science
- 1 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 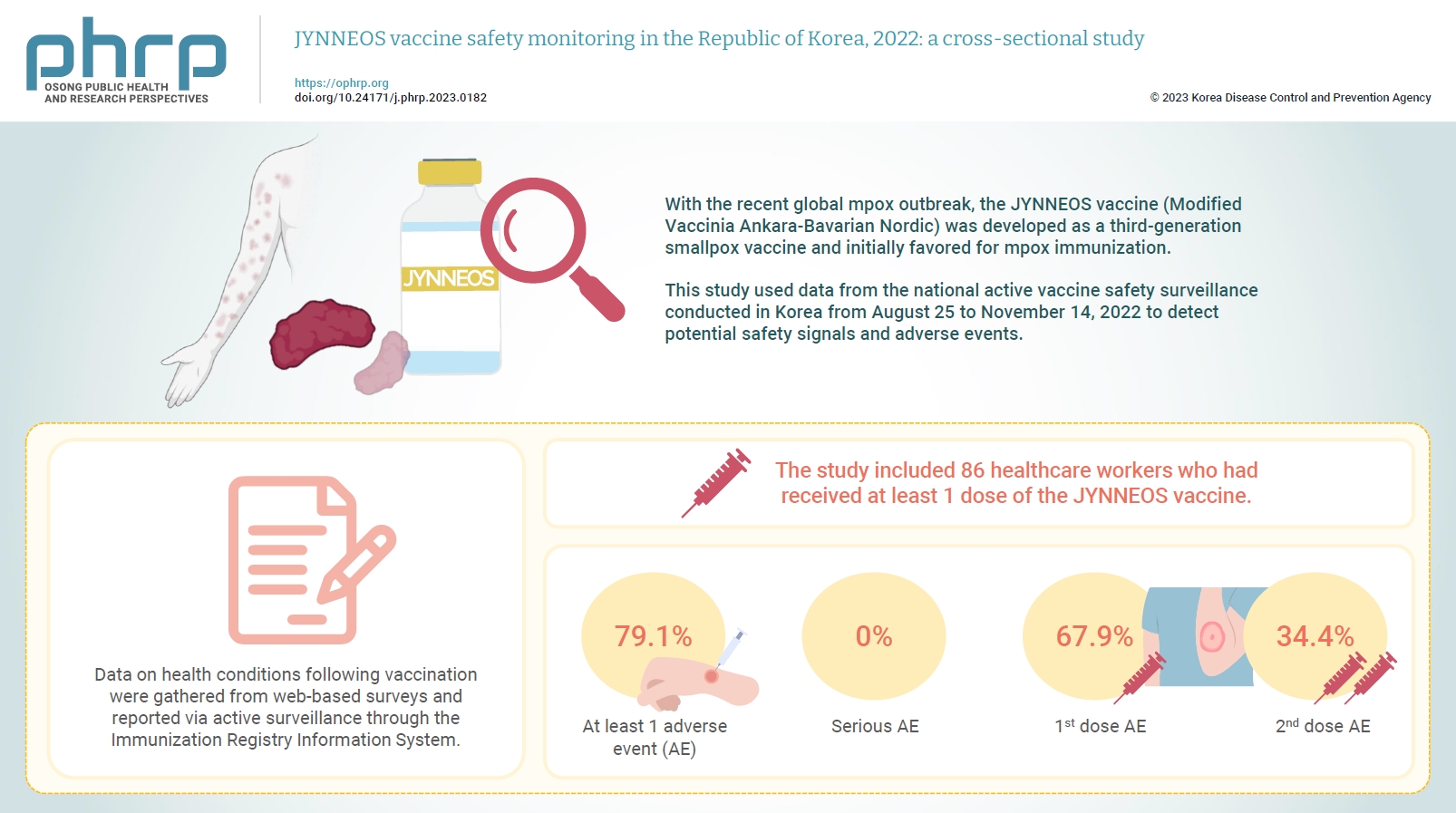
- Objectives
With the recent global mpox outbreak, the JYNNEOS vaccine (Modified Vaccinia Ankara-Bavarian Nordic) was developed as a third-generation smallpox vaccine and initially favored for mpox immunization. Vaccine-associated side effects contribute to vaccine hesitancy. Consequently, tracking adverse events post-immunization is crucial for safety management. This study used data from the national active vaccine safety surveillance conducted in Korea from August 25 to November 24, 2022 to detect potential safety signals and adverse events. Methods: Data on health conditions following vaccination were gathered from web-based surveys and reported via active surveillance through the Immunization Registry Information System. This follow-up system functioned via a text message link, surveying adverse events and health conditions beginning on the second day post-vaccination. Information about specific adverse events, including both local and systemic reactions, was collected. Results: The study included 86 healthcare workers who had received at least 1 dose of the JYNNEOS vaccine. Among the respondents, 79.1% reported experiencing at least 1 adverse event, with the majority being local reactions at the injection site. The incidence of adverse events was higher following the first dose (67.9%) than after the second dose (34.4%). The most frequently reported adverse event for both doses was mild pain at the injection site. Conclusion: The study provides crucial information on the safety of the JYNNEOS vaccine, demonstrating that most adverse events were manageable and predominantly localized to the injection site. Nonetheless, additional research is needed on the safety of various vaccine administration techniques and the vaccine’s effects on broader demographics. -
Citations
Citations to this article as recorded by- Adverse Reactions After Intradermal Vaccination With JYNNEOS for Mpox in Korea
So Yun Lim, Yu Mi Jung, Yeonjae Kim, Gayeon Kim, Jaehyun Jeon, BumSik Chin, Min-Kyung Kim
Journal of Korean Medical Science.2024;[Epub] CrossRef
- Adverse Reactions After Intradermal Vaccination With JYNNEOS for Mpox in Korea
Original Article
- Prevalence and patterns of adverse events following childhood immunization and the responses of mothers in Ile-Ife, South West Nigeria: a facility-based cross-sectional survey
- Olorunfemi Akinbode Ogundele, Funmito Omolola Fehintola, Mubarak Salami, Rahmat Usidebhofoh, Mary Aderemi Abaekere
- Osong Public Health Res Perspect. 2023;14(4):291-299. Published online July 27, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0071
- 2,204 View
- 133 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 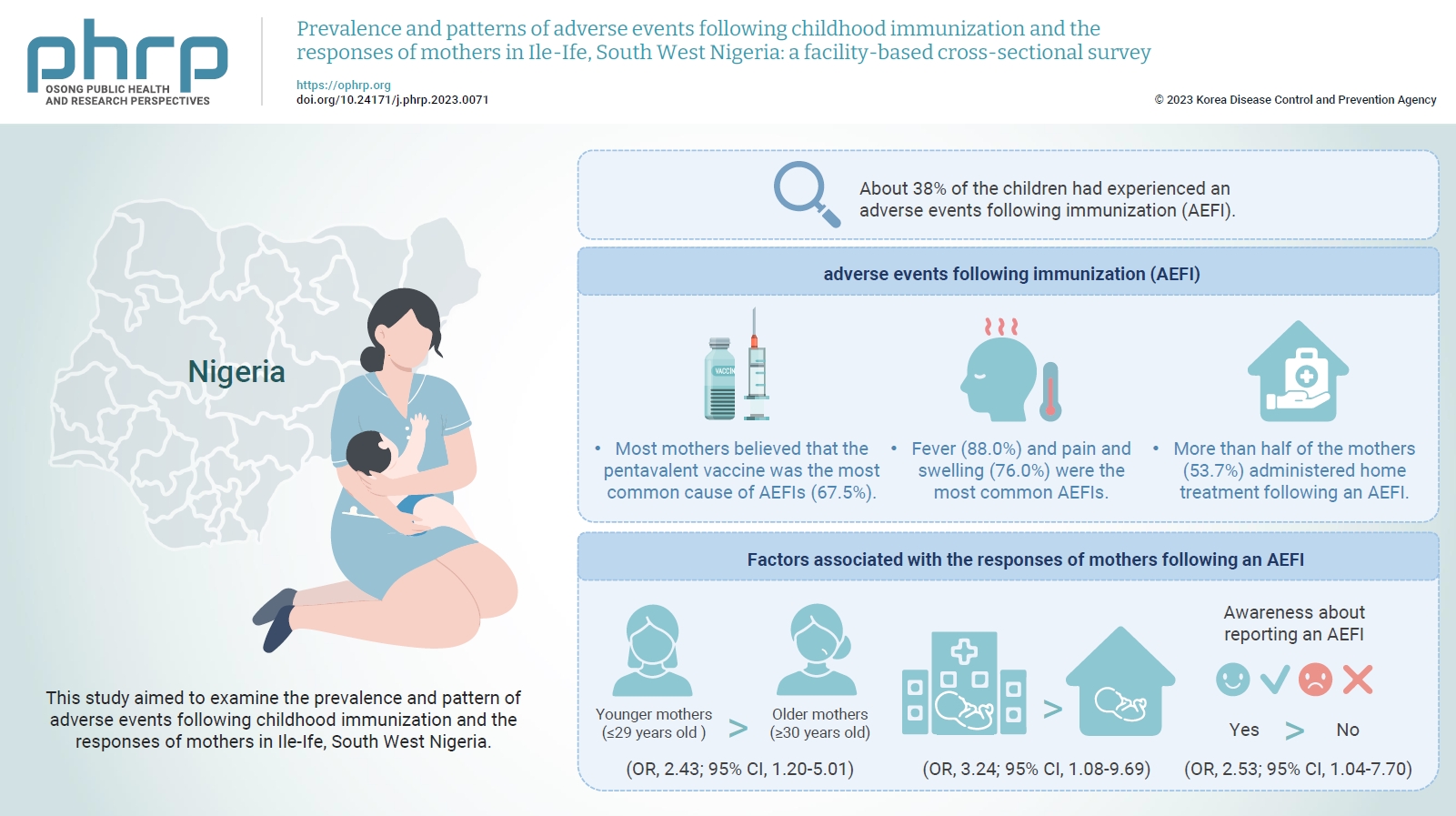
- Objectives
This study aimed to examine the prevalence and pattern of adverse events following childhood immunization and the responses of mothers in Ile-Ife, South West Nigeria.
Methods
This descriptive cross-sectional study was conducted among 422 mothers of children aged 0 to 24 months attending any of the 3 leading immunization clinics in Ile-Ife, Nigeria. The respondents were selected using the multi-stage sampling technique. Data were collected using a pretested structured interviewer-administered questionnaire and analyzed using IBM SPSS ver. 26.0. The chi-square test was used to test associations, while binary logistic regression was used to determine the predictors of mothers’ responses to adverse events following immunization (AEFIs). A p-value of <0.05 was considered statistically significant.
Results
The mean age of the respondents was 29.99±5.74 years. About 38% of the children had experienced an AEFI. Most mothers believed that the pentavalent vaccine was the most common cause of AEFIs (67.5%). Fever (88.0%) and pain and swelling (76.0%) were the most common AEFIs. More than half of the mothers (53.7%) administered home treatment following an AEFI. Younger mothers (odds ratio [OR], 2.43; 95% confidence interval [CI], 1.20–5.01), mothers who delivered their children at a healthcare facility (OR, 3.24; 95% CI, 1.08–9.69), and mothers who were knowledgeable about reporting AEFIs (OR, 2.53; 95% CI, 1.04–7.70) were most likely to respond appropriately to AEFIs.
Conclusion
The proportion of mothers who responded poorly to AEFIs experienced by their children was significant. Therefore, strategies should be implemented to improve mothers’ knowledge about AEFIs to improve their responses.
Brief Report
- Temporal association between the age-specific incidence of Guillain-Barré syndrome and SARS-CoV-2 vaccination in Republic of Korea: a nationwide time-series correlation study
- Hyunju Lee, Donghyok Kwon, Seoncheol Park, Seung Ri Park, Darda Chung, Jongmok Ha
- Osong Public Health Res Perspect. 2023;14(3):224-231. Published online June 22, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0050
- 2,061 View
- 89 Download
- 1 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 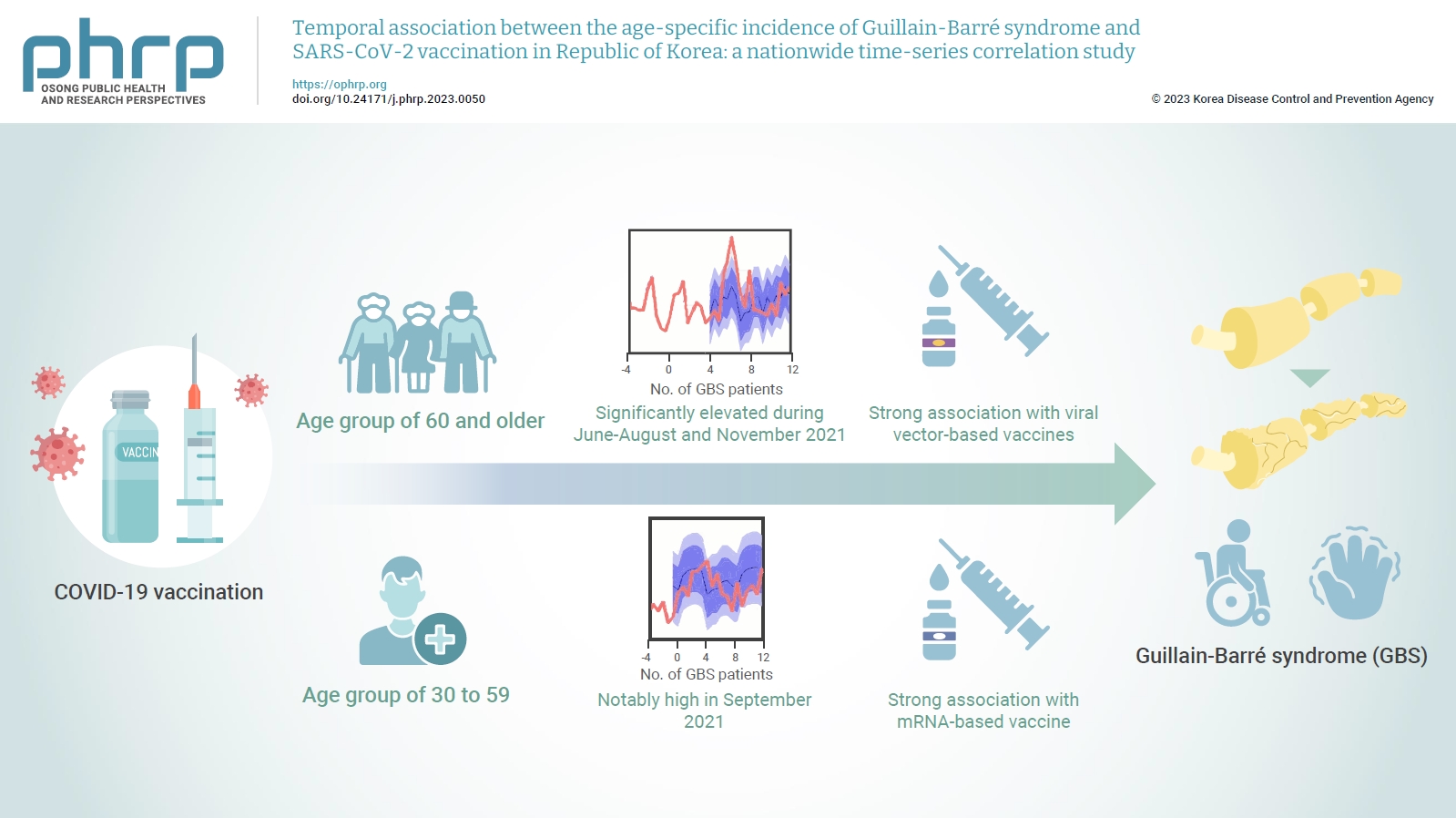
- Objectives
The incidence of Guillain-Barré syndrome (GBS) changed significantly during the coronavirus disease 2019 (COVID-19) pandemic. Emerging reports suggest that viral vector-based vaccines may be associated with an elevated risk of GBS.
Methods
In this nationwide time-series correlation study, we examined the age-specific incidence of GBS from January 2011 to August 2022, as well as data on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccinations and infections from February 2021 to August 2022. We compared the forecasted estimates of age-specific GBS incidence, using the pre-SARS-CoV-2 period as a benchmark, with the actual incidence observed during the post-vaccination period of the pandemic. Furthermore, we assessed the temporal association between GBS, SARS-CoV-2 vaccinations, and COVID-19 for different age groups.
Results
In the age group of 60 and older, the rate ratio was significantly elevated during June-August and November 2021. A significant, strong positive association was observed between viral vector-based vaccines and GBS incidence trends in this age group (r=0.52, p=0.022). For the 30 to 59 years age group, the rate ratio was notably high in September 2021. A statistically significant, strong positive association was found between mRNA-based vaccines and GBS incidence in this age group (r=0.61, p=0.006).
Conclusion
Viral vector-based SARS-CoV-2 vaccines were found to be temporally associated with an increased risk of GBS, particularly in older adults. To minimize age-specific and biological mechanism-specific adverse events, future vaccination campaigns should adopt a more personalized approach, such as recommending homologous mRNA-based SARS-CoV-2 vaccines for older adults to reduce the heightened risk of GBS. -
Citations
Citations to this article as recorded by- mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions
Tamás Bakos, Tamás Mészáros, Gergely Tibor Kozma, Petra Berényi, Réka Facskó, Henriette Farkas, László Dézsi, Carlo Heirman, Stefaan de Koker, Raymond Schiffelers, Kathryn Anne Glatter, Tamás Radovits, Gábor Szénási, János Szebeni
International Journal of Molecular Sciences.2024; 25(7): 3595. CrossRef - Guillain–Barre syndrome following COVID-19 vaccination: a study of 70 case reports
Biki Kumar Sah, Zahra Fatima, Rajan Kumar Sah, Bushra Syed, Tulika Garg, Selia Chowdhury, Bikona Ghosh, Binita Kunwar, Anagha Shree, Vivek Kumar Sah, Anisha Raut
Annals of Medicine & Surgery.2024; 86(4): 2067. CrossRef
- mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions
Original Articles
- Vaccine effectiveness and the epidemiological characteristics of a COVID-19 outbreak in a tertiary hospital in Republic of Korea
- Seonhee Ahn, Tae Jong Son, Yoonsuk Jang, Jihyun Choi, Young Joon Park, Jiseon Seong, Hyun Hee Kwon, Muk Ju Kim, Donghyok Kwon
- Osong Public Health Res Perspect. 2023;14(3):188-196. Published online June 8, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0066
- 1,452 View
- 72 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 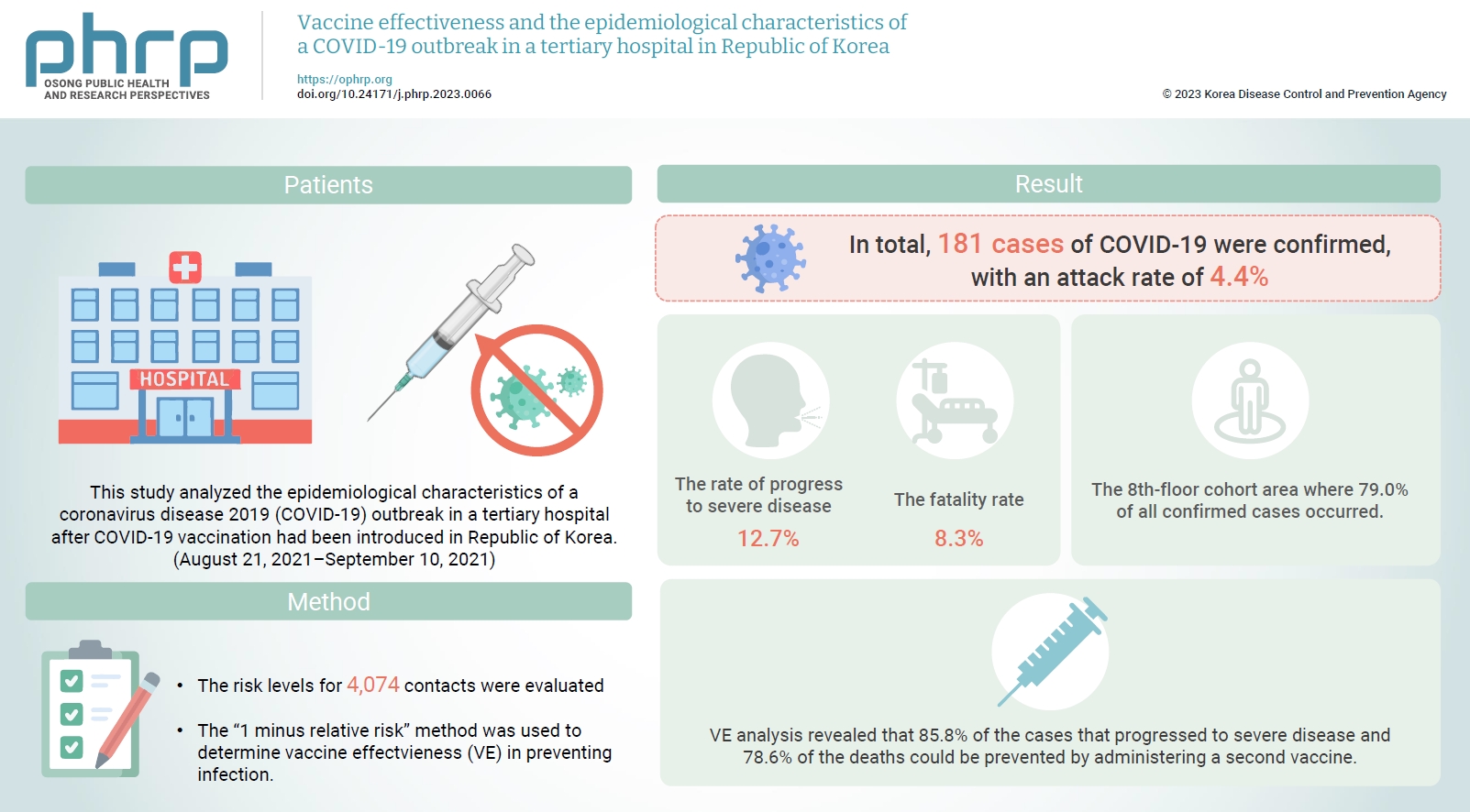
- Objectives
Healthcare facilities are high-risk sites for infection. This study analyzed the epidemiological characteristics of a coronavirus disease 2019 (COVID-19) outbreak in a tertiary hospital after COVID-19 vaccination had been introduced in Republic of Korea. Vaccine effectiveness (VE) and shared anti-infection strategies are also assessed.
Methods
The risk levels for 4,074 contacts were evaluated. The epidemiological characteristics of confirmed cases were evaluated using the chi-square test. The “1 minus relative risk” method was used to determine VE in preventing infection, progression to severe disease, and death. In the largest affected area (the 8th floor), a separate relative risk analysis was conducted. A multivariate logistic regression analysis (with 95% confidence interval [CIs]) was used to identify transmission risk factors with a significance level <10% via the backward elimination method.
Results
In total, 181 cases of COVID-19 were confirmed, with an attack rate of 4.4%. Of those cases, 12.7% progressed to severe disease, and 8.3% died. In the cohort isolation area on the 8th floor, where 79.0% of the confirmed cases occurred, the adjusted odds ratio was 6.55 (95% CI, 2.99–14.33) and 2.19 (95% CI, 1.24–3.88) for caregivers and the unvaccinated group, respectively. VE analysis revealed that 85.8% of the cases that progressed to severe disease and 78.6% of the deaths could be prevented by administering a second vaccine.
Conclusion
Caregiver training for infection prevention and control is necessary to reduce infection risk. Vaccination is an important intervention to reduce the risk of progression to severe disease and death.
- Effectiveness of the COVID-19 vaccine in the Honam region of the Republic of Korea
- In-Sook Shin, Yong-Pyo Lee, Seung-Hoon Lee, Jae-Young Lee, Jong-Ha Park, Yoon-Seok Chung
- Osong Public Health Res Perspect. 2023;14(3):197-206. Published online June 8, 2023
- DOI: https://doi.org/10.24171/j.phrp.2022.0308
- 1,852 View
- 63 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 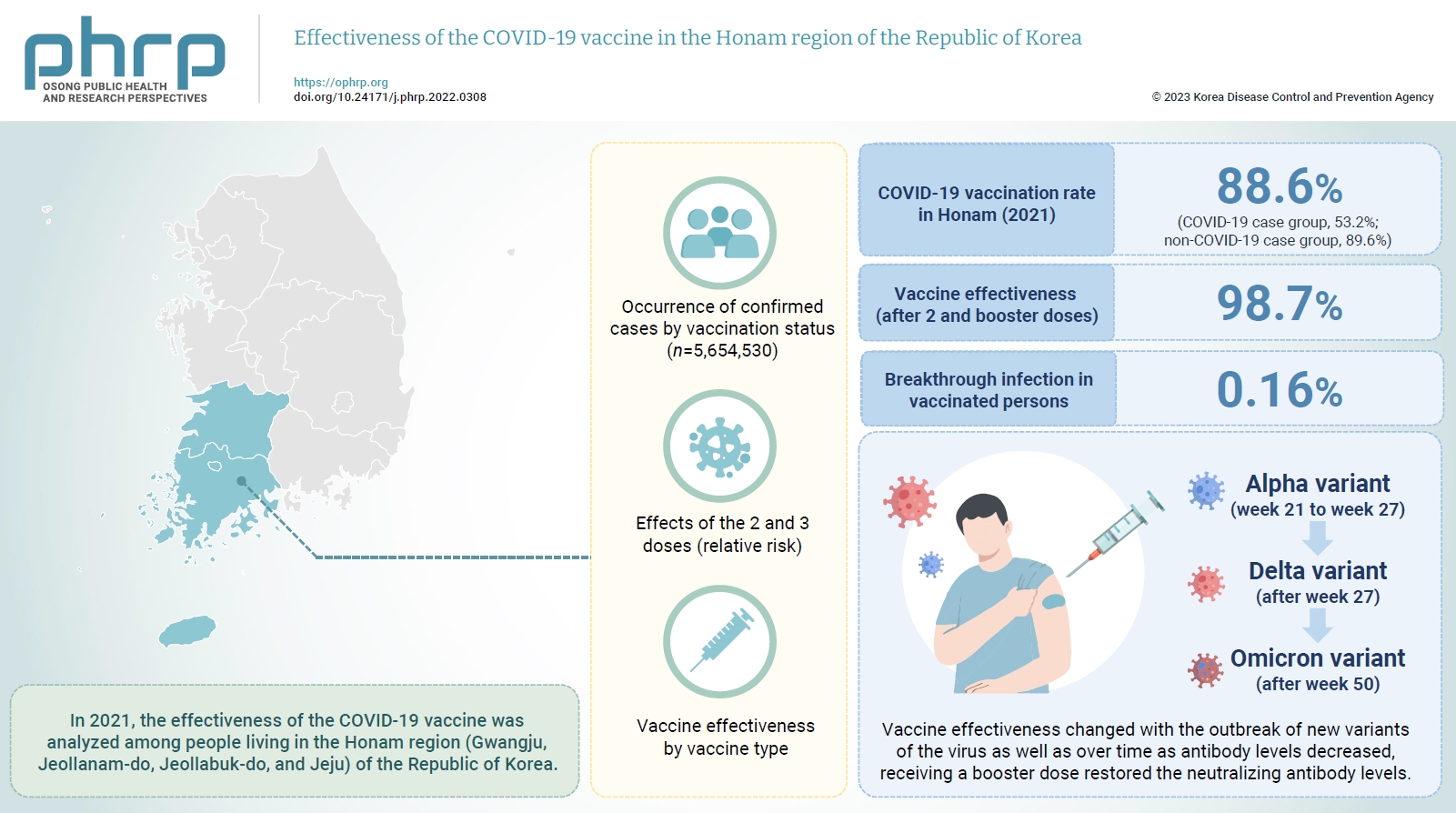
- Objectives
In 2021, the effectiveness of the COVID-19 vaccine was analyzed among people living in the Honam region (Gwangju, Jeollanam-do, Jeollabuk-do, and Jeju) of the Republic of Korea. And we investigated changes in the dominant virus strain.
Methods
This study used the data provided by the Korean Ministry of the Interior and Safety for individuals ≥12 years old in the Honam region, and the Integrated Disease and Health Management System of the Korea Centers for Disease Control and Prevention for COVID-19-vaccinated individuals as of December 31, 2021. Statistical analyzes were performed using IBM SPSS ver. 23.0. The occurrence of confirmed cases by vaccination status, the relative risk, and vaccine effectiveness by vaccine type were calculated.
Results
In 2021, the COVID-19 vaccination rate in Honam was 88.6%. The overall vaccine effectiveness (after 2 and 3 doses) was 98.7% (p<0.001). and the breakthrough infection rate was 0.16%. From week 21 to week 27 of 2021 (June 27 to July 3), the genome sequencing results were mostly alpha variants. The Delta variant emerged as the dominant variant after 27 weeks and the Omicron variant was found at 50 weeks (December 5–11).
Conclusion
Vaccine effectiveness changed with the outbreak of new variants of the virus as well as over time as antibody levels decreased. that the prevention effectiveness of vaccination in Honam was >98%, and the effect among persons who received 2 doses was >90% regardless of the vaccine type. Although vaccine effectiveness decreased because of reduced antibody levels over time (as observed in breakthrough infections), receiving a booster dose restored the neutralizing antibody levels.
- The incidence and clinical characteristics of myocarditis and pericarditis following mRNA-based COVID-19 vaccination in Republic of Korea adolescents from July 2021 to September 2022
- Ju-Young Sim, Seung-Yun Kim, Eun-Kyoung Kim
- Osong Public Health Res Perspect. 2023;14(2):76-88. Published online April 18, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0032
- 3,934 View
- 231 Download
- 2 Web of Science
- 2 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 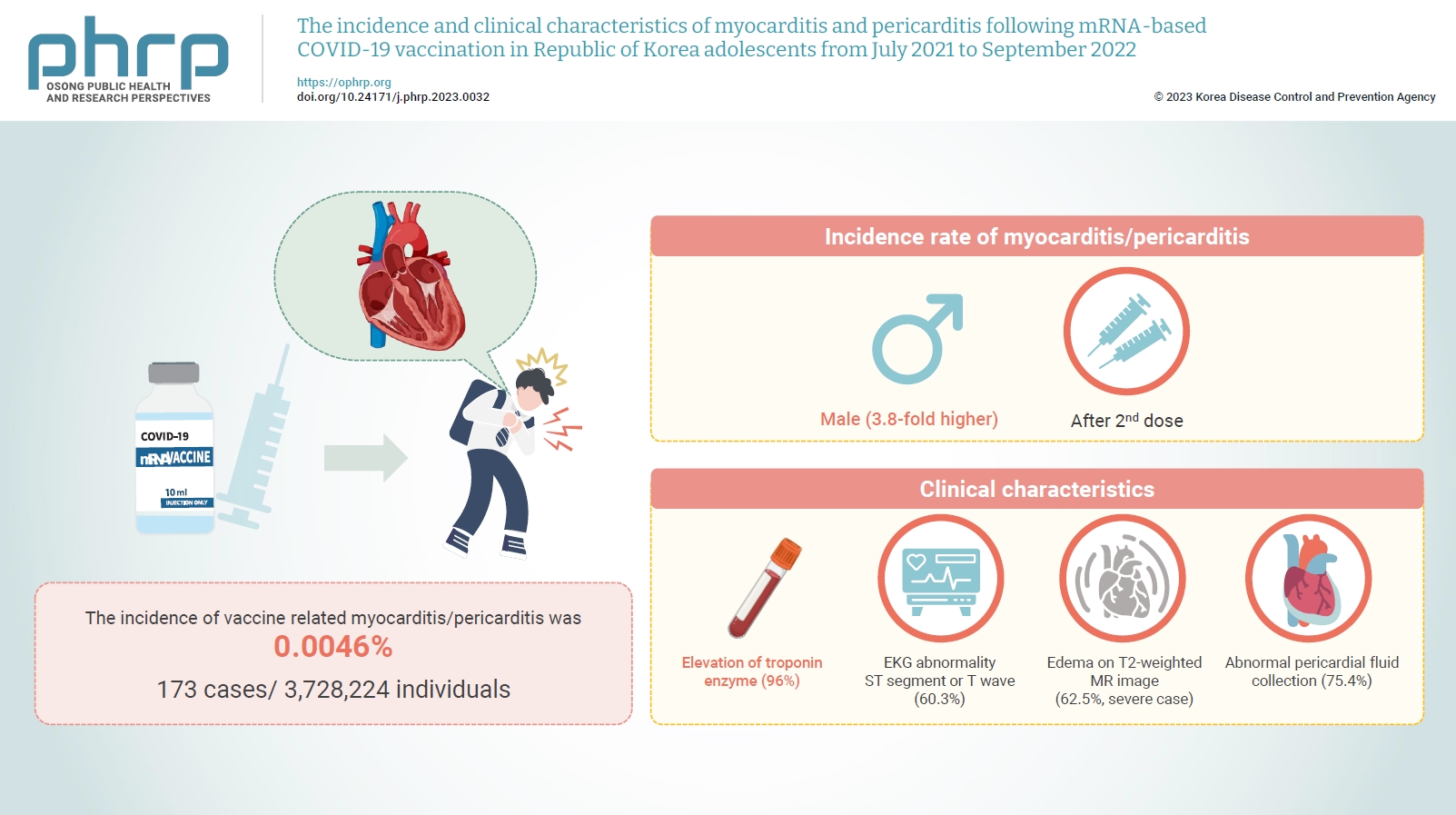
- Objectives
Age-specific information regarding myocarditis/pericarditis in adolescents following mRNA-based coronavirus disease 2019 (COVID-19) vaccination in Asia remains insufficient. This study investigated the incidence and clinical characteristics of myocarditis/pericarditis in Republic of Korea adolescents after mRNA-based COVID-19 vaccination.
Methods
This retrospective descriptive study utilized patient data from the Korea Immunization Management System. Incidence rates were calculated according to age and sex. Clinical characteristics (symptoms/signs, laboratory values, and imaging results) were compared between mild and severe cases.
Results
Between July 19, 2021 and September 30, 2022, 3,728,224 individuals aged 12 to 19 years received 6,484,165 mRNA-based COVID-19 vaccines, and 173 cases met the case definition for myocarditis/pericarditis: 151 mild (87.3%) and 22 severe (12.7%). The incidence was 3.8-fold higher in males than in females. Troponin I/ troponin T was elevated in 96% of myocarditis cases, demonstrating higher sensitivity than creatine kinase-myocardial band (67.6%) or C-reactive protein (75.2%). ST-segment or Twave on electrography abnormalities were found in 60.3% (85/141). Paroxysmal/sustained atrial/ventricular arrhythmias were more common in severe than in mild cases (45.5% vs. 16.8%, p=0.008). Edema on T2-weighted magnetic imaging occurred in 21.6% (8/37) and 62.5% (5/8) of mild and severe cases, respectively (p=0.03). Abnormal pericardial fluid collection or pericardial inflammation was found in 75.4% of pericarditis cases (49/65).
Conclusion
Myocarditis/pericarditis occurred in rare cases following mRNA-based COVID-19 vaccination. Most cases were mild, but the incidence was higher in adolescent males and after the second dose. As bivalent severe acute respiratory syndrome coronavirus 2 mRNA vaccination started in Republic of Korea in October 2022, the post-vaccination incidence of myocarditis/pericarditis should be closely monitored, considering clinical characteristics. -
Citations
Citations to this article as recorded by- Responses to Common Misconceptions Relating to COVID-19 Variant-Adapted mRNA Vaccines
George Kassianos, Pauline MacDonald, Ivan Aloysius, Shanti Pather
Vaccines.2024; 12(1): 57. CrossRef - To become a more stronger and safer country
Jong-Koo Lee
Osong Public Health and Research Perspectives.2023; 14(2): 67. CrossRef
- Responses to Common Misconceptions Relating to COVID-19 Variant-Adapted mRNA Vaccines
- COVID-19 outbreak in a religious village community in Republic of Korea and risk factors for transmission
- Jiae Shim, Eunju Lee, Eunyoung Kim, Yeonhwa Choi, Giseok Kang, Bryan Inho Kim
- Osong Public Health Res Perspect. 2023;14(2):110-118. Published online April 5, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0002
- 1,491 View
- 72 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Objectives
This study aimed to assess the scale and transmission patterns of coronavirus disease 2019 (COVID-19) in a religious village community in South Korea, to determine the risk factors of transmission, and to evaluate vaccine effectiveness.
Methods
An epidemiological survey was conducted, and data were collected and analyzed from 602 villagers in the religious village community. Multivariate logistic regression analysis was used to identify the risk factors for COVID-19 transmission and to evaluate vaccine effectiveness.
Results
The outbreak attack rate was 72.1% (434/602). The attack rate was high among women in their 60s, the unemployed, residents living near religious facility (<500 m), and the unvaccinated. Age, the distance between religious facility and residences, and the absence of vaccination were identified as risk factors for transmission. Vaccine effectiveness was 49.0%, and the highest effectiveness was seen in the age group of 59 years or younger (65.8%).
Conclusion
This village community was isolated, with little communication with the outside world. However, the frequency of close contact between residents was relatively high, contributing to the spread of COVID-19 in the village even with relatively short exposure. Vaccination rates in the village community were also lower than those in the general public. Public health authorities should consider the potential impact of cultural factors, including religion, that could lead to the exponential spread of COVID-19 in closed village communities.
Special Article
- A framework for nationwide COVID-19 vaccine safety research in the Republic of Korea: the COVID-19 Vaccine Safety Research Committee
- Na-Young Jeong, Hyesook Park, Sanghoon Oh, Seung Eun Jung, Dong-Hyun Kim, Hyoung-Shik Shin, Hee Chul Han, Jong-Koo Lee, Jun Hee Woo, Byung-Joo Park, Nam-Kyong Choi
- Osong Public Health Res Perspect. 2023;14(1):5-14. Published online February 28, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0026
- 3,011 View
- 153 Download
- 4 Web of Science
- 3 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- With the introduction of coronavirus disease 2019 (COVID-19) vaccines, the Korea Disease Control and Prevention Agency (KDCA) commissioned the National Academy of Medicine of Korea to gather experts to independently assess post-vaccination adverse events. Accordingly, the COVID-19 Vaccine Safety Research Committee (CoVaSC) was launched in November 2021 to perform safety studies and establish evidence for policy guidance. The CoVaSC established 3 committees for epidemiology, clinical research, and communication. The CoVaSC mainly utilizes pseudonymized data linking KDCA’s COVID-19 vaccination data and the National Health Insurance Service’s claims data. The CoVaSC’s 5-step research process involves defining the target diseases and organizing ad-hoc committees, developing research protocols, performing analyses, assessing causal relationships, and announcing research findings and utilizing them to guide compensation policies. As of 2022, the CoVaSC completed this research process for 15 adverse events. The CoVaSC launched the COVID-19 Vaccine Safety Research Center in September 2022 and has been reorganized into 4 divisions to promote research including international collaborative studies, long-/short-term follow-up studies, and education programs. Through these enhancements, the CoVaSC will continue to swiftly provide scientific evidence for COVID-19 vaccine research and compensation and may serve as a model for preparing for future epidemics of new diseases.
-
Citations
Citations to this article as recorded by- Risk of encephalitis and meningitis after COVID-19 vaccination in South Korea: a self-controlled case series analysis
Ju Hwan Kim, Dongwon Yoon, Hwa Yeon Ko, Kyungyeon Jung, Jun-Sang Sunwoo, Won Chul Shin, Jung-Ick Byun, Ju-Young Shin
BMC Medicine.2024;[Epub] CrossRef - To become a more stronger and safer country
Jong-Koo Lee
Osong Public Health and Research Perspectives.2023; 14(2): 67. CrossRef - Risk of lymphadenopathy from SARS-CoV-2 vaccination in Korea: a self-controlled case series analysis
Mi-Sook Kim, Bongyoung Kim, Jeong Pil Choi, Nam-Kyong Choi, Jung Yeon Heo, Jun Yong Choi, Joongyub Lee, Sang Il Kim
Epidemiology and Health.2023; 45: e2023090. CrossRef
- Risk of encephalitis and meningitis after COVID-19 vaccination in South Korea: a self-controlled case series analysis
Brief Report
- The effectiveness of Paxlovid treatment in long-term care facilities in South Korea during the outbreak of the Omicron variant of SARS-CoV-2
- Hanul Park, Young Joon Park, Hye Young Lee, Mi Yu, Yeong-Jun Song, Sang Eun Lee, Ji-Joo Lee, Eun-Sol Lee, Yeonjung Kim
- Osong Public Health Res Perspect. 2022;13(6):443-447. Published online December 23, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0262
- 3,147 View
- 215 Download
- 7 Web of Science
- 9 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 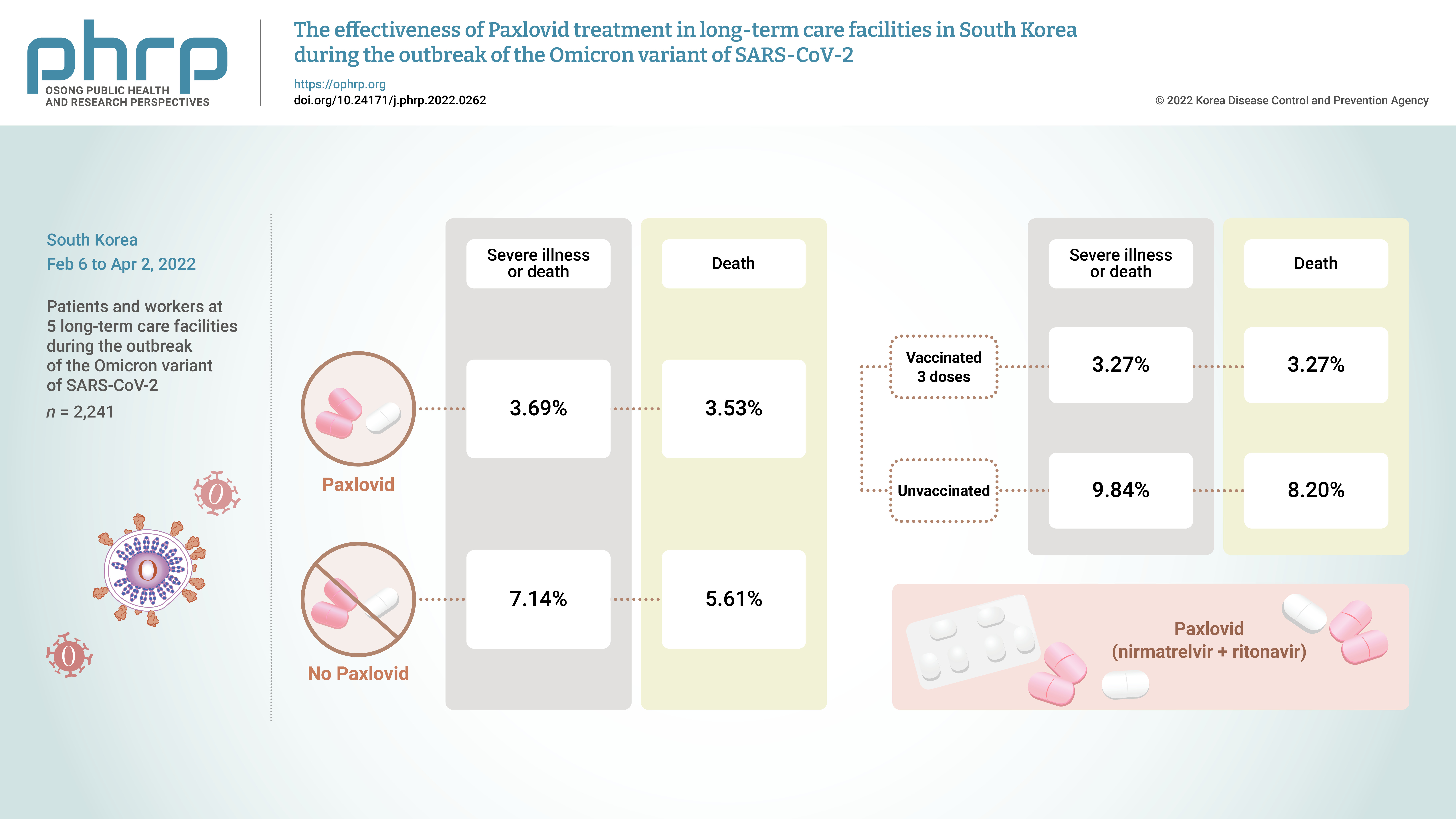
- Objectives
On November 5, 2021, Pfizer Inc. announced Paxlovid (nirmatrelvir +ritonavir) asa treatment method that could reduce the risk of hospitalization or death for patients withconfirmed coronavirus disease 2019 (COVID-19).Methods: From February 6, 2022 to April 2, 2022, the incidence of COVID-19 and the effectsof treatment with Paxlovid were analyzed in 2,241 patients and workers at 5 long-term carefacilities during the outbreak of the Omicron variant of severe acute respiratory syndromecoronavirus 2 in South Korea.Results: The rate of severe illness or death in the group given Paxlovid was 51% lower thanthat of the non-Paxlovid group (adjusted risk ratio [aRR], 0.49; 95% confidence interval [CI],0.24−0.98). Compared to unvaccinated patients, patients who had completed 3 doses of thevaccine had a 71% reduced rate of severe illness or death (aRR, 0.29; 95% CI, 0.13−0.64) and a65% reduced death rate (aRR, 0.35; 95% CI, 0.15−0.79).Conclusion: Patients given Paxlovid showed a lower rate of severe illness or death and alower fatality rate than those who did not receive Paxlovid. Patients who received 3 dosesof the vaccine had a lower rate of severe illness or death and a lower fatality rate than theunvaccinated group. -
Citations
Citations to this article as recorded by- Efficacy and safety of antiviral treatments for symptomatic COVID-19 outpatients: Systematic review and network meta-analysis
Meital Zur, Thalia Peselev, Stav Yanko, Victoria Rotshild, Ilan Matok
Antiviral Research.2024; 221: 105768. CrossRef - Clinical Effectiveness of Ritonavir-Boosted Nirmatrelvir—A Literature Review
Sydney Paltra, Tim O. F. Conrad
Advances in Respiratory Medicine.2024; 92(1): 66. CrossRef - Effectiveness of nirmatrelvir‐ritonavir on severe outcomes of COVID‐19 in the era of vaccination and Omicron: An updated meta‐analysis
Sien Ombelet, Diego Castanares‐Zapatero, Fabian Desimpel, Frank Hulstaert, Sabine Stordeur, Dominique Roberfroid
Journal of Medical Virology.2024;[Epub] CrossRef - COVID‐19 infection in patients with haematological malignancies: A single‐centre survey in the latest Omicron wave in China
Xiaolu Zhu, Qian Jiang, Jin Lu, Yuqian Sun, Xiaosu Zhao, Shenmiao Yang, Feifei Tang, Wenjing Yu, Ting Zhao, Xiaohong Liu, Jinsong Jia, Wenbing Duan, Lijuan Hu, Jing Wang, Yang Liu, Nan Peng, Xuelin Dou, Rui Ma, Qiang Fu, Huifang Wang, Kaiyan Liu, Xiaojun
British Journal of Haematology.2023; 202(1): 31. CrossRef - The association mental health of adolescents with economic impact during the COVID-19 pandemic: a 2020 Korean nationally representative survey
Hanul Park, Kang-Sook Lee
BMC Public Health.2023;[Epub] CrossRef - Efficacy and safety of paxlovid (nirmatrelvir/ritonavir) in the treatment of COVID‐19: An updated meta‐analysis and trial sequential analysis
Haokun Tian, Changsen Yang, Tiangang Song, Kechen Zhou, Lequan Wen, Ye Tian, Lirui Tang, Weikai Xu, Xinyuan Zhang
Reviews in Medical Virology.2023;[Epub] CrossRef - Real-World Effectiveness of Nirmatrelvir-Ritonavir and Its Acceptability in High-Risk COVID-19 Patients
Min-Kyung Kim, Kyung-Shin Lee, Sin Young Ham, Youn Young Choi, Eunyoung Lee, Seungjae Lee, Bora Lee, Jaehyun Jeon, BumSik Chin, Yeonjae Kim, Gayeon Kim, Hee-Chang Jang, Jae-Phil Choi, Sang-Won Park
Journal of Korean Medical Science.2023;[Epub] CrossRef - Effectiveness of Molnupiravir Treatment in Patients with COVID-19 in Korea: A Propensity Score Matched Study
Hye Rim Park, Min-Gyu Yoo, Jong Mu Kim, Soon Jong Bae, Hyungmin Lee, Jungyeon Kim
Infection & Chemotherapy.2023; 55(4): 490. CrossRef - Nirmatrelvir combined with ritonavir for preventing and treating COVID-19
Stefanie Reis, Maria-Inti Metzendorf, Rebecca Kuehn, Maria Popp, Ildiko Gagyor, Peter Kranke, Patrick Meybohm, Nicole Skoetz, Stephanie Weibel
Cochrane Database of Systematic Reviews.2023;[Epub] CrossRef
- Efficacy and safety of antiviral treatments for symptomatic COVID-19 outpatients: Systematic review and network meta-analysis
Original Article
- mRNA vaccine effectiveness against SARS-CoV-2 B.1.617.2 (Delta) and B.1.1.529 (Omicron) variant transmission from home care cases to household contacts in South Korea
- Hanul Park, Young Joon Park, Sang Eun Lee, Min Jei Lee, Hyungtae Ahn
- Osong Public Health Res Perspect. 2022;13(6):435-442. Published online November 28, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0243
- 4,732 View
- 171 Download
- 1 Web of Science
- 1 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 
- Objectives
Household contacts of confirmed cases of coronavirus disease 2019 (COVID-19) areexposed to a high risk of viral transmission, and secondary incidence is an important indicatorof community transmission. This study analyzed the secondary attack rate and mRNA vaccineeffectiveness against transmission (VET) for index cases (patients treated at home) confirmedto be infected with the Delta and Omicron variants.Methods: The subjects of the study were 4,450 index cases and 10,382 household contacts.Logistic regression analysis was performed to compare the secondary attack rate byvaccination status, and adjusted relative risk and 95% confidence intervals were identified.Results: The secondary attack rate of the Delta variant was 27.3%, while the secondary attackrate of the Omicron variant was 29.8%. For the Delta variant, groups with less than 90 daysand more than 90 days after 2 doses of mRNA vaccination both showed a VET of 37%. For theOmicron variant, a 64% VET was found among those with less than 90 days after 2 doses ofmRNA vaccination.Conclusion: This study provides useful data on the secondary attack rate and VET of mRNAvaccines for household contacts of COVID-19 cases in South Korea. -
Citations
Citations to this article as recorded by- Household secondary attack rates and risk factors during periods of SARS-CoV-2 Delta and Omicron variant predominance in the Republic of Korea
Jin Lee, Mijeong Ko, Seontae Kim, Dosang Lim, Gemma Park, Sang-Eun Lee
Osong Public Health and Research Perspectives.2023; 14(4): 263. CrossRef
- Household secondary attack rates and risk factors during periods of SARS-CoV-2 Delta and Omicron variant predominance in the Republic of Korea



 First
First Prev
Prev


