Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 3(4); 2012 > Article
-
Articles
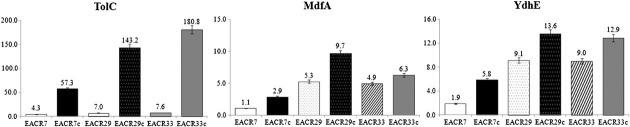
Resistance to Fluoroquinolone by a Combination of Efflux and Target Site Mutations in EnteroaggregativeEscherichia coli Isolated in Korea - Jun-Young Kim, Se-Mi Jeon, Hyungjun Kim, Nara Lim, Mi-Sun Park, Seong-Han Kim
-
Osong Public Health and Research Perspectives 2012;3(4):239-244.
DOI: https://doi.org/10.1016/j.phrp.2012.11.002
Published online: December 31, 2012
Division of Enteric Diseases, Korea National Institute of Health, Osong, Korea.
- Corresponding author. E-mail: kkingsh@chol.com
• Received: October 19, 2012 • Accepted: October 25, 2012
Copyright ©2012, Korea Centers for Disease Control and Prevention
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Detection of gyrA and parC Mutations and Prevalence of Plasmid-Mediated Quinolone Resistance Genes in Klebsiella pneumoniae
Sawsan Mohammed Kareem, Israa MS Al-kadmy, Saba S Kazaal, Alaa N Mohammed Ali, Sarah Naji Aziz, Rabab R Makharita, Abdelazeem M Algammal, Salim Al-Rejaie, Tapan Behl, Gaber El-Saber Batiha, Mohamed A El-Mokhtar, Helal F Hetta
Infection and Drug Resistance.2021; Volume 14: 555. CrossRef - Fluoroquinolone-Transition Metal Complexes: A Strategy to Overcome Bacterial Resistance
Mariana Ferreira, Paula Gameiro
Microorganisms.2021; 9(7): 1506. CrossRef - Frequency of DNA gyrase and topoisomerase IV mutations and plasmid-mediated quinolone resistance genes among Escherichia coli and Klebsiella pneumoniae isolated from urinary tract infections in Azerbaijan, Iran
Robab Azargun, Mohammad Hossein Soroush Barhaghi, Hossein Samadi Kafil, Mahin Ahangar Oskouee, Vahid Sadeghi, Mohammad Yousef Memar, Reza Ghotaslou
Journal of Global Antimicrobial Resistance.2019; 17: 39. CrossRef - Evaluating the efficacy of an algae-based treatment to mitigate elicitation of antibiotic resistance
Kassandra L. Grimes, Laura J. Dunphy, Erica M. Loudermilk, A. Jasmin Melara, Glynis L. Kolling, Jason A. Papin, Lisa M. Colosi
Chemosphere.2019; 237: 124421. CrossRef - Resistance mechanisms of Helicobacter pylori and its dual target precise therapy
Yuehua Gong, Yuan Yuan
Critical Reviews in Microbiology.2018; 44(3): 371. CrossRef - E. coli Group 1 Capsular Polysaccharide Exportation Nanomachinary as a Plausible Antivirulence Target in the Perspective of Emerging Antimicrobial Resistance
Shivangi Sachdeva, Raghuvamsi V. Palur, Karpagam U. Sudhakar, Thenmalarchelvi Rathinavelan
Frontiers in Microbiology.2017;[Epub] CrossRef - Plasmid-Mediated Quinolone Resistance in Different Diarrheagenic Escherichia coli Pathotypes Responsible for Complicated, Noncomplicated, and Traveler's Diarrhea Cases
Silvia Herrera-León, María Teresa Llorente, Sergio Sánchez
Antimicrobial Agents and Chemotherapy.2016; 60(3): 1950. CrossRef - Determinants of quinolone resistance in Escherichia coli causing community-acquired urinary tract infection in Bejaia, Algeria
Yanat Betitra, Vinuesa Teresa, Viñas Miguel, Touati Abdelaziz
Asian Pacific Journal of Tropical Medicine.2014; 7(6): 462. CrossRef


 PubReader
PubReader Cite
Cite
