Evaluation of Potency on Diphtheria and Tetanus Toxoid for Adult Vaccines by In Vivo Toxin Neutralization Assay Using National Reference Standards
Article information
Abstract
Objectives
Vaccinations against diphtheria and tetanus are essential in providing immunity against these bacterial infections. The potency of diphtheria and tetanus toxoid vaccines can be measured using the in vivo toxin neutralization assay. The limit of potency of this assay was determined only for children. Therefore, we assessed the potency of adult vaccines using this assay to identify the feasibility of limit for adult vaccines.
Methods
Fifteen lots of tetanus-reduced diphtheria and tetanus-diphtheria-acellular pertussis vaccines were used. In vivo toxin neutralization and lethal challenge assays were conducted on each vaccine to calculate the potencies of the toxoids. National reference standards for toxins and antitoxins were used for in vivo toxin neutralization assay.
Results
All 15 lots satisfied the limits of potency for lethal challenge assay. The potency of diphtheria and tetanus toxoids exceeded 1 and 8 units/mL, respectively, for in vivo toxin neutralization assay.
Conclusion
Although additional studies are required for new assays and limits, the current level of potency for adult vaccines as determined by in vivo toxin neutralization assay, was demonstrated in this study. Such efforts to improve assays are expected to promote the development of diphtheria and tetanus vaccines for adults and to contribute to vaccine self-sufficiency.
Introduction
Although diphtheria and tetanus are very rare in developed countries, they are still sporadically reported in young children, the elderly, and unvaccinated populations. Diphtheria is caused by Corynebacterium diphtheria infections and is due to the toxins produced by these bacteria. The infection is spread through the respiratory system and causes pharyngeal infection, myocarditis, polyneuropathy, and systemic toxicity [1,2]. Tetanus is caused by the neurotoxins produced by Clostridium tetani. Often present in the soil, these bacteria can proliferate in scars on the skin. The symptoms of tetanus include muscle spasm, contraction, difficulty in breathing, high blood pressure, and seizure [1,3].
Immunoglobulins or antibiotics can be administered to treat diphtheria and tetanus. However, the most effective preventative measure is to administer a vaccine containing diphtheria and tetanus toxoids. Diphtheria-tetanus-acellular pertussis (DTaP), tetanus-reduced diphtheria (Td), and tetanus-diphtheria-acellular pertussis (Tdap) vaccines are currently administered in Korea.
Diphtheria and tetanus toxoid vaccine potency may be measured using the lethal challenge, intradermal challenge, in vivo toxin neutralization, and vero cell assays [4]. In Korea, Japan, and Europe, the lethal challenge and the vero cell assays are used for national lot release of Td and Tdap vaccines. Also, the in vivo toxin neutralization assay is used for the DTaP vaccine. In the lethal challenge assay, lethal amounts of toxins are subcutaneously injected into animals (guinea pigs for diphtheria and mice for tetanus). The amount of vaccine that can protect against this lethal dose, and the amount of the reference standard with the same protective effects are compared, to calculate potency and are adjusted into international units (IU) [5]. However, the lethal challenge assay requires large numbers of animals (100 guinea pigs for diphtheria toxoid and 116 mice for tetanus toxoid). In contrast, the in vivo toxin neutralization assay requires approximately 1/10 of the animals used in the lethal challenge assay and is easier to set up and perform than the vero cell assay. However, only the limit of potency for the children’s vaccine has been established for the in vivo toxin neutralization assay, but no limit has been determined for the adult vaccine.
Considering that it is essential to prepare assays to measure the potency of toxoids for vaccine development, the lethal challenge assay, which requires many animals, could hinder the development of vaccines. Therefore, the present study conducted in vivo toxin neutralization assays in various lots of Td and Tdap vaccines for adults using diphtheria and tetanus toxin and antitoxin with national reference standards (NRS) from the Ministry of Food and Drug Safety (MFDS). The level of potency for adult vaccines was assessed, and the possibility of replacing lethal challenge assay with in vivo toxin neutralization assay was examined.
Materials and Methods
1. Vaccines and reference standards
A total of 15 lots of vaccines were used, including 6 lots of Td vaccine for adults (6 lots from manufacturer MC) and 9 lots of Tdap vaccines for adults (4 lots from manufacturer MC, 3 lots from manufacturer MS, and 2 lots from manufacturer MP).
For reference standards to be used in diphtheria and tetanus toxoid potency tests, the following NRS established by the MFDS were used: diphtheria toxin (MFDS, 07/017), diphtheria antitoxin (MFDS, 07/018), tetanus toxin (MFDS, 01/001), and tetanus antitoxin (MFDS, 10/036). Moreover, diphtheria toxoid [adsorbed (National Institute for Biological Standards and Control, 07-216)] was used as the reference standard for absorbed diphtheria toxoid vaccine, and tetanus vaccine [adsorbed (European Directorate for the Quality of Medicines, T0400000)] was used as the reference standard for absorbed tetanus toxoid vaccine.
2. Lethal challenge assay for diphtheria toxoid
For each group, 10 Hartley guinea pigs (Orient, Seongnam, Korea) weighing 250–350 g were used. Four groups were administered the reference standards whilst 4 other groups received vaccines. To measure LD50, 4 groups of 5 animals were used. The absorbed diphtheria toxoid vaccine reference standard was dissolved in physiological saline at a concentration of 4 IU/mL. The vaccine reference standard and vaccine were then serially diluted in physiological saline in 4 steps at a dilution factor of 1.7. The vaccine reference standard and vaccine were subcutaneously injected in 1 mL. After 28 days, 1 mL of dilution of diphtheria toxin (100 LD50/mL) was subcutaneously injected, and survival was monitored for 4 days. For the diphtheria toxin LD50 measurement group, the toxin dilutions (100 LD50/mL) additionally diluted ×10, ×50, ×250, and ×1,250 were subcutaneously injected in 1 mL, and animal survival was monitored for 4 days. The test results were statistically analyzed through probit analysis on Bioassay 2.0.0 (MFDS, Osong, Korea), and the potency was calculated.
3. In vivo toxin neutralization assay for diphtheria toxoid
For animal immunization, 1 group of 5 Hartley guinea pigs (Orient, Seongnam, Korea) weighing 450–550 g was used. Vaccine (0.5 mL) was subcutaneously injected, and the animals were observed for 5 weeks. After 5 weeks, blood was collected from the heart using 10 mL syringes, and serum was separated. Equal amounts of serum collected from each guinea pig were pooled together. The pooled serum and diphtheria toxin (1 L+/mL) were mixed in ratios of 1:1 and 1:2 and neutralized for 1 hour at room temperature. Here, the solution produced by combining diphtheria toxin (1 L+/mL) and diphtheria antitoxin (1 IU/mL) in a 1:1 ratio was used as the control. Neutralized pooled serum with diphtheria toxin (2 mL) was injected subcutaneously into 2 guinea pigs weighing 240–280 g, and the guinea pigs were monitored for 4 days for survival. The ratios that resulted in later deaths than that of the control group were confirmed, and the vaccine potencies were calculated from the ratios.
4. Lethal challenge assay for tetanus toxoid
For each group, 16 Institute of Cancer Research (ICR) mice (Orient, Seongnam, Korea) weighing 17–20 g were used. Three groups were administered the reference standards while 3 other groups received vaccines. To measure LD50, 4 groups of 5 animals were used. The absorbed tetanus toxoid vaccine reference standard was dissolved in physiological saline at a concentration of 2.5 IU/mL. The vaccine reference standard and vaccine were then serially diluted in physiological saline in 3 steps at a dilution factor of 2. The vaccine reference standard and vaccine were subcutaneously injected in 0.5 mL. After 28 days, 0.5 mL of dilution of tetanus toxin (100 LD50/0.5 mL) was subcutaneously injected, and their survival was monitored for 4 days. For the tetanus toxin LD50 measurement group, the toxin dilutions (100 LD50/0.5 mL) additionally diluted ×10, ×50, ×250, and ×1,250 were subcutaneously injected in 0.5 mL, and the survival was monitored for 4 days. The test results were statistically analyzed through probit analysis on Bioassay 2.0.0 (MFDS, Osong, Korea), and the potency was calculated.
5. In vivo toxin neutralization assay for tetanus toxoid
Animal immunization was conducted identically to the in vivo toxin neutralization assay for diphtheria toxoid. Pooled serum and tetanus toxin (0.5 L+/mL) were combined in ratios of 1:8 and 1:16, and the mixtures were neutralized for 1 hour at room temperature. Here, the solution produced by combining tetanus toxin (0.5 L+/mL) and tetanus antitoxin (0.5 IU/mL) in a ratio of 1:1 was used as the control. After neutralization, 0.4 mL of the neutralized pooled serum with tetanus toxin was injected subcutaneously into ICR mice (Orient, Seongnam, Korea) weighing 16–20 g, and the animals’ survival was monitored for 4 days. The ratios that resulted in later deaths than that of the control group were confirmed, and the vaccine potencies were calculated from the ratios.
6. Limit of potency for diphtheria and tetanus toxoid
Using the “minimum requirements for biologics (Korea)” as a reference, 2 and 20 IU/0.5 mL, which are limits of potency for diphtheria and tetanus toxoids for adult vaccine, respectively, were used in lethal challenge assays [4].
Results
1. Potency determination of diphtheria toxoid
1.1. Lethal challenge assay
When the potency of diphtheria toxoid was calculated using the lethal challenge assay, all 15 vaccine lots had potencies that exceeded the limit of potency for diphtheria toxoid for the adult vaccine at 2 IU/0.5 mL (Table 1). The potency was higher for the Td vaccine than for the Tdap vaccine (Figure 1A). The LD50 of diphtheria toxin was 112, thus within the acceptable range of 50–200 (results not shown).
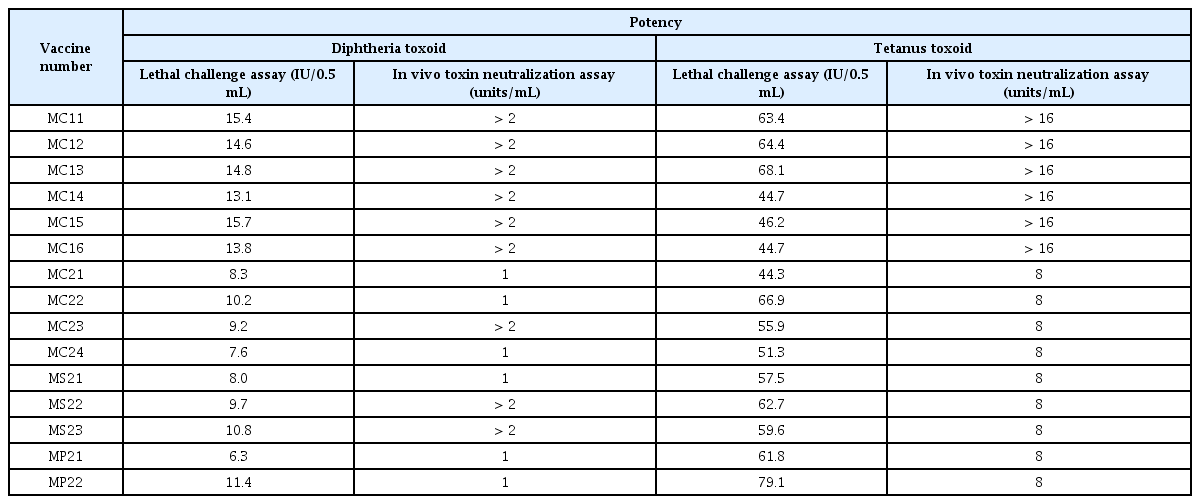
Results of potency estimation of diphtheria and tetanus toxoid by lethal challenge and in vivo toxin neutralization assays.

Comparison of the results of lethal challenge and in vivo toxin neutralization assays for diphtheria toxoid. (A) When the potency of diphtheria toxoid was calculated through lethal challenge assay, all 15 vaccine lots had appropriate potencies satisfying the limits for adult vaccines of 2 IU/0.5 mL. (B) The results of in vivo toxin neutralization assay showed potencies exceeding 1 unit/mL for all vaccine lots.
1.2. In vivo toxin neutralization assay
When the potency of diphtheria toxoid was calculated from the in vivo toxin neutralization assay, all 15 lots showed potencies more than 1 unit/mL (Table 1). As in the lethal challenge assay, the potency was higher for the Td vaccine than the Tdap vaccine (Figure 1B). In the control group where the diphtheria antitoxin was used, all animals died within 96 hours (results not shown).
2. Potency determination of tetanus toxoid
2.1. Lethal challenge assay
When the potency of tetanus toxoid was measured using the lethal challenge assay, all 15 lots had potencies that exceeded the limit of potency for tetanus toxoid for the adult vaccine set at 20 IU/0.5 mL (Table 1). No difference in potency was observed between Td and Tdap vaccines in the lethal challenge assay (Figure 2A). The LD50 of tetanus toxin was 112, thus within the acceptable range of 50–200 (results not shown).
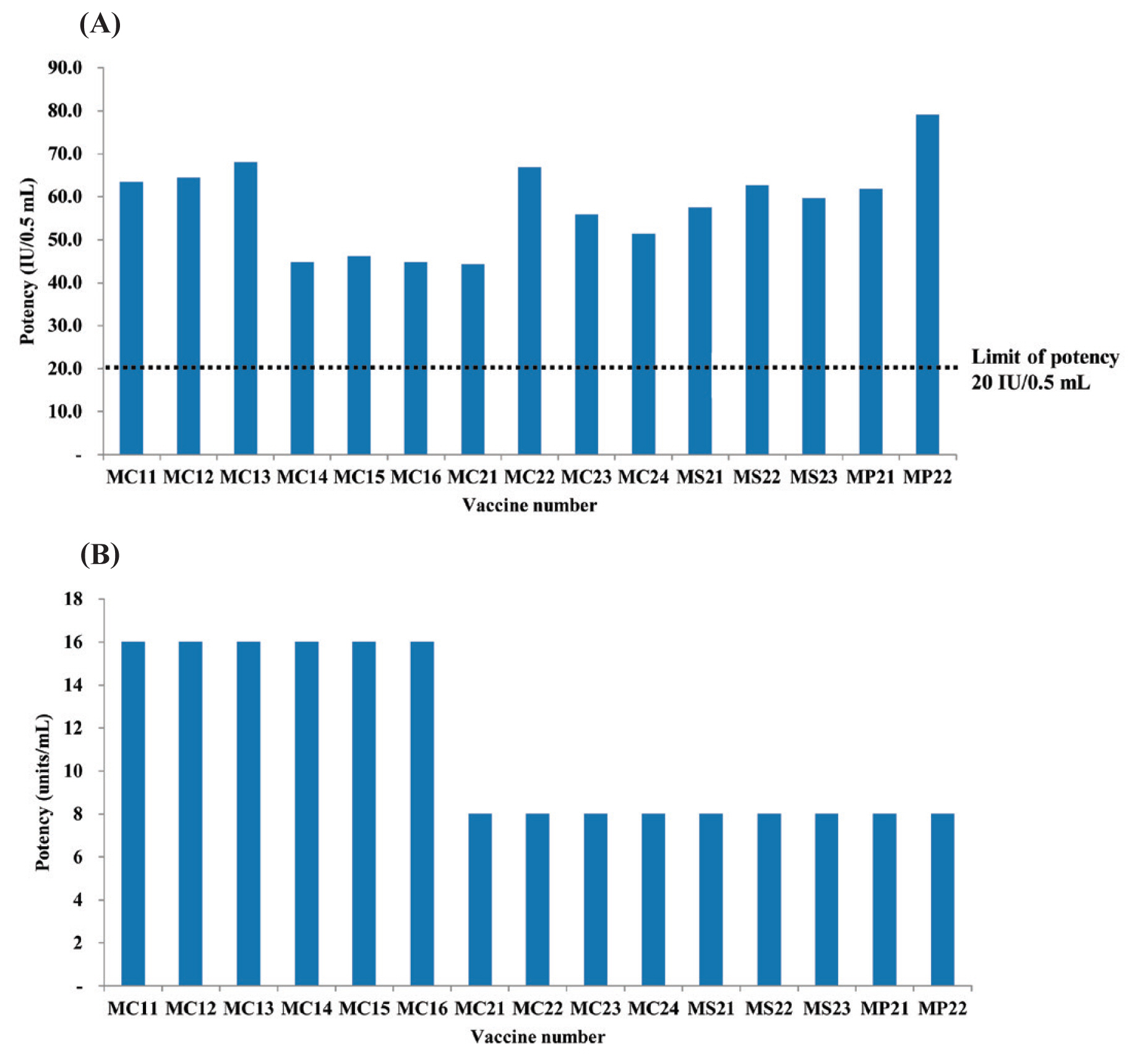
Comparison of the results of lethal challenge and in vivo toxin neutralization assays for tetanus toxoid. (A) When the potency of tetanus toxoid was calculated through lethal challenge assay, all 15 vaccine lots had appropriate potencies satisfying the limits for adult vaccines of 20 IU/0.5 mL. (B) The results of in vivo toxin neutralization assay showed potencies exceeding 8 units/mL for all vaccine lots.
2.2. In vivo toxin neutralization assay
When the potency of tetanus toxoid was calculated from the in vivo toxin neutralization assay, all 15 lots showed potencies of more than 8 units/mL (Table 1). The potency was higher for the Td vaccine than for the Tdap vaccine (Figure 2B). In the control group where the tetanus antitoxin was used, all animals died within 96 hours (results not shown).
Discussion
Since the introduction of the DTaP vaccine in Korea in 1982, high vaccination rates have resulted in diphtheria infection being a very rare occurrence. Tetanus has been reported in 10–20 cases per year since the 2000’s [6]. However, due to the increasing numbers of immigrants and visitors from other countries where different disease profiles and vaccination rates may differ from Korea, it is important to continue to prevent these 2 diseases in high-risk groups [7].
It is very rare to acquire immunity to diphtheria and tetanus through natural infection, and vaccination is the only known effective preventative measure against the occurrence of these diseases [8]. In general, antibodies produced through DTaP vaccination in childhood decrease gradually into adulthood, therefore, re-vaccination with Td or Tdap vaccines is necessary [9]. In Korea, all babies and children receive DTaP or DTaP combined inactivated poliovirus (DTaP-IPV) vaccines through the National Immunization Program. When children reach 11 or 12 years old, Td or Tdap vaccines are offered again, and booster vaccinations are provided every 10 years thereafter [10].
The quality of vaccines containing diphtheria and tetanus toxoids is assured through the national lot release by the MFDS, before commercialization in Korea. At this step, the potency of toxoid in the vaccine is confirmed. For the in vivo toxin neutralization assay, only limits for children’s diphtheria and tetanus toxoids vaccines have been set (2 units/mL) [4]. The MFDS has established NRS whose potencies have been calculated in comparison to the World Health Organization international reference standards. The established NRS for diphtheria and tetanus toxins and antitoxins are used in national lot release and quality monitoring [11].
Although the lethal challenge assay is regularly used in potency tests, the assay requires many animals. This is in contrast to the in vivo toxin neutralization assay that requires only a small number of animals making it simpler and more ethical. When the potencies of 15 vaccine lots used in the present study were measured using the lethal challenge assay, all potencies satisfied the limits indicating use of in vivo toxin neutralization assay. The same vaccines were used for the in vivo toxin neutralization assay for diphtheria toxoids where the potencies of all vaccines exceeded 1 unit/mL, and for tetanus toxoids where potencies of all vaccines exceeded 8 units/mL. Although combination vaccines require fewer immunizations than single vaccines, they can show various changes in safety and efficacy profiles [12]. In particular, for the DTaP vaccine, acellular pertussis may lower the immunogenicity of diphtheria toxoid [13]. Also, in the present study the Tdap vaccine combining diphtheria, tetanus, and pertussis, had generally lower activities for diphtheria and tetanus toxoids than the Td vaccine that combines diphtheria and tetanus. This observation was clearer in the in vivo toxin neutralization assay.
The present study used various lots of vaccines, produced by various manufacturers to investigate the feasible limit of potency for adult vaccines. Moreover, the study also showed that the in vivo toxin neutralization assay using NRS was comparable to and potentially could replace the lethal challenge assay, which has been recognized as an obstacle in the development of vaccines for adults containing diphtheria and tetanus toxoids. Further studies are needed to validate these findings and aid the development of diphtheria and tetanus vaccines specifically for adults in Korea.
Notes
Conflicts of Interest
The authors have declared no conflicts of interest.