Impact of Cognitive Aging on Health-Related Quality of Life in Menopausal Women
Article information
Abstract
Objectives
Menopause is a well-known risk factor for accelerating cognitive aging in women. This study aimed to assess differences in cognitive function and health-related quality of life (HRQOL) according to menopausal status to determine whether the menopause significantly affects the relationship between cognitive function and HRQOL.
Methods
This was a cross-sectional comparative study with a convenience sample of 178 Korean women including 89 naturally menopausal women (65 ± 10 years) and 89 non-menopausal women (45 ± 8 years) who met the eligibility criteria and completed neuropsychological tests and self-report questionnaires about their HRQOL, cognitive function, depression, and sleep quality. Multiple regression analyses were performed within and between groups according to menopausal status.
Results
Menopausal women had significantly worse scores on neuropsychological performance and HRQOL than non-menopausal women. A better neuropsychological performance (β = 0.34) was solely associated with a better HRQOL in menopausal women, whilst socioeconomic variables were associated with HRQOL in non-menopausal women.
Conclusion
Menopause is an important risk factor for HRQOL, and the association between cognition and HRQOL may differ according to menopausal status. When developing programs for target groups to improve daily functioning and HRQOL, healthcare professionals need to pay more attention to this relationship.
Introduction
Cognitive aging is a normal process of aging where there is a gradual decline in cognition beginning from early adulthood [1]. Age-related decline in specific cognitive ability domains are well documented. Fluid cognition including psychomotor, speed of processing, memory, and executive function have been reported to diminish gradually at an estimated rate of −0.02 standard deviations per year, compared to crystalized cognition which slightly improved at an estimated rate of 0.0003 to 0.02 standard deviations per year [2]. According to a 27-year longitudinal study with 2,225 participants living in the community, aged from 31 to 99 years, a significant decline in cognition occurred at about 65 years and accelerated after 80 years [3]. Significantly, patterns of cognitive decline were similar between older males and females (60–80 years) as measured by standard neuropsychological tests, although men performed better on visuospatial ability test, whereas women performed better on an episodic memory test [4].
Menopause is a well-recognized risk factor that may accelerate cognitive aging in women [5]. In middle-aged women, increasing age and the menopause accelerated cognitive decline in processing speed, episodic memory, and working memory [6]. Longitudinal declines were also reported in processing speed and verbal memory [6]. This suggests that the menopause may lead to an increase in women’s vulnerability to cognitive decline from middle age, and a continuous decline in specific domains of cognition, in addition to the effects of chronological aging.
Cognitive decline even when mild, can interfere with effective functioning in everyday life and health-related quality of life (HRQOL) in elderly women (≥ 65 years) [7]. In previous studies, both self-reported and objectively measured cognitive function had a negative impact on global, as well as specific domains of HRQOL, and this influence remained after controlling for confounding factors including socio-demographic characteristics, health-related habits, and health conditions [8,9]. Interestingly, executive functions including attention and working memory which were measured prospectively over 1 year in menopausal women living in the community, had an independent association with HRQOL [10]. However, this finding did not provide satisfactory evidence for a difference in the relationship between cognitive function and HRQOL based on the independent effects of chronological age and menopausal status in a cognitively intact female population.
Adverse changes in menopausal women’s mood and quality of sleep have been reported as distressing symptoms that women may experience due to the menopause and aging [11–13]. In the general population, nearly 1/3 of people experience sleep problems [14] and at least 43% of older people (≥ 65 years) living in the community experience sleep problems [15]. The prevalence rate in the female population who experience sleep problems ranged from 16% to 42% in the non-menopausal women, compared to 35% to 60% in postmenopausal women [16]. There were 6.7% of a sample of the general population in Korea (n = 4,949) that felt depressed, with 4.2% of men and 9.1% of women [17]. Similar to sleeping problems, depression is more prevalent in the older population aged ≥ 60 years (7.3% to 11.2%), compared to young and middle-aged populations (3.8% to 8.2%) [17]. It is well known that the relationship between feeling depressed and having disturbed sleep is bi-directional, and these problems have negative impacts on the physical, emotional, mental, and social domains of HRQOL [18,19]. Sleep and mood status should be considered as important factors to better reflect the relationship between cognitive function and HRQOL [20].
Theoretically, attention and working memory processes are necessary for executive functions such as goal-directed behavior, problem solving, and social functioning [21]. These cognitive processes are required to maintain and promote health-related behaviors including learning about new health-related information, self-monitoring of physical conditions, health-related habit changes, and communication with others such as healthcare professionals [9,22]. Understanding this relationship has important implications for developing effective strategies to optimize daily functioning, improving HRQOL, and assisting menopausal women to manage cognitive decline whilst maintaining a healthy status. Thus, this study aims to assess cognitive function and HRQOL in menopausal women to examine whether the menopause significantly affects the relationship between cognitive function and HRQOL after controlling for possible covariates such as depression and sleep problems.
Materials and Methods
1. Study design and sample
This was a cross-sectional comparative study with a convenience sample of 178 Korean women including 89 naturally menopausal women and 89 non-menopausal women. All participants were recruited from regional health promotion centers in D city from 2015 to 2016. Eligible participants were female, at least 20 years old, able to read and speak Korean and provide written informed consent, and had a Mini-Mental State Examination score above a cut-off point indicating cognitive impairment such as dementia [23]. In this study menopause was defined as absence of menstruation for 1 year or longer. Menopausal women had a history of amenorrhea for the last 12 months while non-menopausal women did not meet this criteria. Women experiencing the menopause induced by surgery or other medical reasons were excluded from the study. Additionally, participants were excluded who had preexisting mental, psychiatric, or learning disorders that may affect their ability to perform neuropsychological tests. The sample size for this study was calculated using power analysis as determined by the G*Power program Version 3.1 [24]. It was determined that 120 participants were required to power the study (85% power), to detect a medium effect size for mean differences in the outcome variables between 2 groups at the 5% level of significance. A sample size of 120 participants was determined to be sufficient to power (85%) a medium effect size with 8 predictors, at a 5% level of significance in a multiple linear regression analysis [9,10]. There were 178 participants enrolled in this study.
2. Procedure and ethical considerations
This study was approved by the Institutional Review Board of Chungnam National University (no.: 2-1046881-A-N-01-201510-HR-047). Data collection were conducted by research assistants who were trained by a neuroscientist. A brief explanation of the research objectives, confidentiality, potential risks and benefits, and other ethical considerations was provided to potential participants, and then detailed information was given to women who decided to participate in this study. Informed consent was obtained from all participants prior to initiation of the study. It took about 30 minutes to complete neuropsychological tests and self-report questionnaires in a quiet place at the center.
3. Measurements
Neuropsychological tests and self-report questionnaires were used to assess HRQOL, cognitive function, depression, and overall sleep quality. Demographic and health-related characteristics were also measured as possible covariates.
3.1. Outcome variable
3.1.1. HRQOL
The EuroQoL 5 dimensions questionnaire (EQ-5D) is a standardized instrument for measuring HRQOL [25]. This measure comprises of 5 questions on mobility, self-care, usual activities, pain, and psychological status, with 3 possible answers for each item. A total score was derived from these 5 questions by applying the conversion used for the Korean population [26]. The maximum score of 1 indicates the best level of HRQOL, and higher scores show higher values for HRQOL with less severe or frequent problems. The Korean version of the EQ-5D was confirmed as a valid and reliable tool for assessing HRQOL [27].
3.2. Predictor variables
3.2.1. Digit span test
The Digit Span test is a standard neuropsychological test used to measure attention and working memory function [28]. This test comprises of Digit Span Forward (DSF) and Backward (DSB) tests. The DSF test requires the individual to repeat a series of digits in the same order that has been read by the examiner, and the DSB test involves recalling a sequence of digits in reverse order, which requires more cognitive effort. These tests were scored as the number of digits correctly repeated before 2 failed trials.
3.2.2. Trail making test
The Trail Making test (TMT) is a neuropsychological assessment based on a time-tested technique and it is used to measure divided attention, cognitive flexibility, and executive function [28]. In Part A of the TMT, participants were asked to connect the circles of 25 consecutive numbers in sequential order, and in Part B, they were asked to draw lines to connect consecutively numbered and lettered circles, by alternating between numbers and letters. The score represents the amount of time required to complete Parts A and B of the test.
3.2.3. Stroop color-word (Stroop) test
The Stroop test is a color-word naming test, and was used to measure the various cognitive domains including selective attention, inhibitory function, and executive function [29]. The Stroop test consisted of 2 tasks: reading the word and naming the color of the word. When participants were asked to name the color in which a word was printed rather than read the color name, they required a longer time and had more errors during the task. The score denotes the number of items correctly repeated in 2 minutes.
3.2.4. Attentional Function Index
The Attentional Function Index was used as a self-report measure to assess an individual’s perceived effectiveness in performing daily life activities requiring attention, working memory, and executive function [30]. This measure consists of 13 items where each item is measured on a 10-point scale. Higher scores indicated more effective cognitive functioning at the time of administration. The reliability and validity of this instrument has been established [30]. The Cronbach’s alpha was 0.85 in this study, indicating satisfactory reliability.
3.3. Control variables
3.3.1. Patient Health Questionnaire
The 8-item Patient Health Questionnaire (PHQ) is a diagnostic scale that has been established as a valid measure for depressive symptoms in population-based studies [31]. In this questionnaire the individual is asked to assign a score for each item based upon their experience over the last 2 weeks where 0 represents not at all, and 3 reflects a problem they experience nearly every day. The total score ranged from 0 to 24, with higher scores indicating a greater severity of depressive symptoms. The convergent validity of the Korean-translated PHQ was confirmed by significant correlations with the Geriatric Depression Scale and Center for Epidemiological Studies Depression Scale, which are common instruments used to assess the clinical severity of depression [32]. The Cronbach’s alpha coefficient was 0.90 in this study.
3.3.2. Pittsburgh Sleep Quality Index
The Pittsburgh Sleep Quality Index is a self-report measure that assesses quality and patterns of sleep, and includes 7 components of subjective quality: latency, duration, habitual efficiency, disturbance, use of medications to aid sleeping, and daytime dysfunction [33]. The overall sleep score is calculated by adding the scores of the 7 components and ranges from 0 to 21. Higher scores indicate a poorer sleep quality, and a total score of 5 and above is indicative of poor sleep quality. Acceptable reliability, and validity of the Korean-translated sleep quality index measure were obtained in Korean patients [34]. For this study, the Cronbach’s alpha was 0.73, indicating acceptable reliability.
3.3.3. Descriptive variables
Sociodemographic characteristics (age, education level, marital status, and occupational status), health condition (comorbidities and menopausal status), and health-related habits (smoking status, alcohol consumption, and physical activity) were assessed as covariates. Regarding health habits, smoking status was categorized as currently smoking, former smoker, and non-smoker. An individual defined as currently smoking would have smoked at least 100 cigarettes and be actively smoking. A former smoker was defined as an individual having smoked at least 100 cigarettes in the past but no longer smoking at the time of assessment. A non-smoker was defined as an individual who has smoked less than 100 cigarettes in the past [35]. Alcohol consumption status was divided into 3 categories which were currently drinking (drunk alcohol at least once per week and still drinking), a past drinker (drunk alcohol at least once per week but stopped drinking during the last year) and have never drank alcohol (had 12 drinks or less in the past) [36]. Physical activity was defined as the frequency of moderate and intense levels of exercise per week.
4. Statistical analysis
Data were analyzed using IBM SPSS Version 24 for Windows (IBM Corp., Armonk, NY, USA). A Total Cognitive Function Index (TCFI) was computed to create a variable that indicates an objectively measured cognitive function. The TCFI was scored by subtracting the z scores of TMT Part A and TMT Part B, from the sum of z scores of DSF, DSB, Stroop test A, and Stroop test B. Z scores on neuropsychological tests were calculated by using the mean and standard deviation of all participants. Higher scores indicated better cognitive performance. Descriptive statistics analyzed socio-demographic characteristics, health condition, and health habits to describe the sample. The Independent t test was used to compare cognitive function, depressed mood, overall sleep quality, and HRQOL between groups based on menopause status. Pearson’s correlation and other comparative analyses were performed to assess possible covariates associated with HRQOL. Multiple regression analyses were performed to examine the effect of cognitive function on HRQOL after controlling for possible covariates, and to assess whether there is a difference in this relationship according to menopausal status.
Results
1. Demographic characteristics
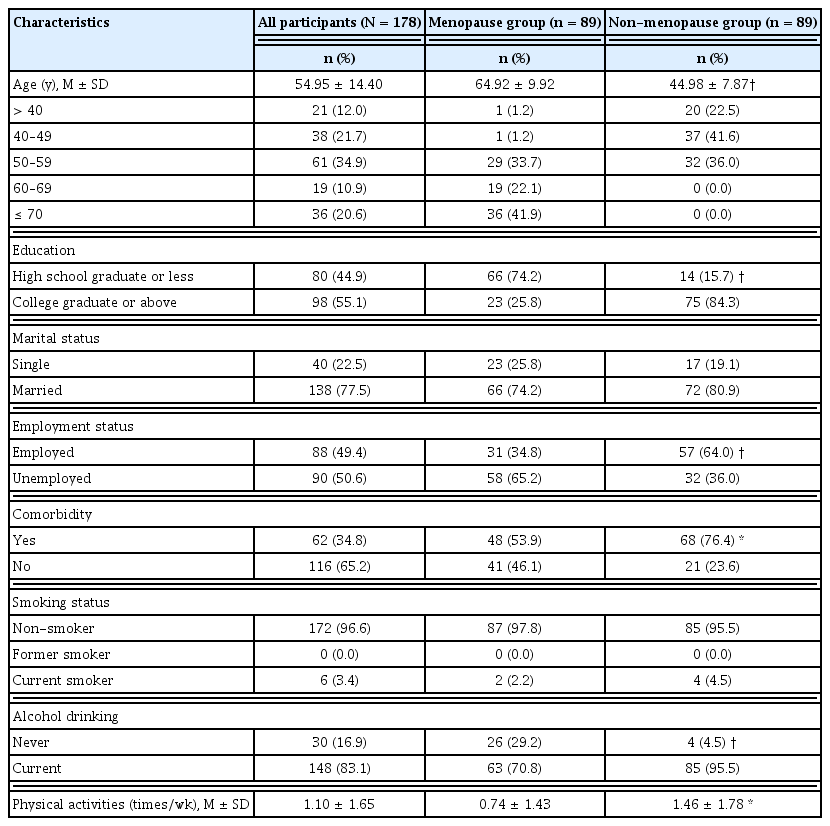
Demographic characteristics are presented in Table 1. The groups statistically significantly differed in age, education, employment status, comorbidity, alcohol consumption, and physical activities. As anticipated, women in the menopause group were older and less educated than those in the non-menopause group (p < 0.001) and the numbers of unemployed (p < 0.001), and presence of comorbidities (p < 0.01) were significantly higher in the menopause group than in the non-menopause group. Regarding health-related habits, more than 95% of women in each group were non-smokers. The number of women currently drinking significantly differed between groups, with 70.8% in the menopause group and 95.5% in the non-menopause group (p < 0.001). The frequency of physical activities was significantly higher in the non-menopause group than in the counterpart group (p < 0.01).
2. Group differences in cognition, depression, sleep, and HRQOL based upon menopausal status
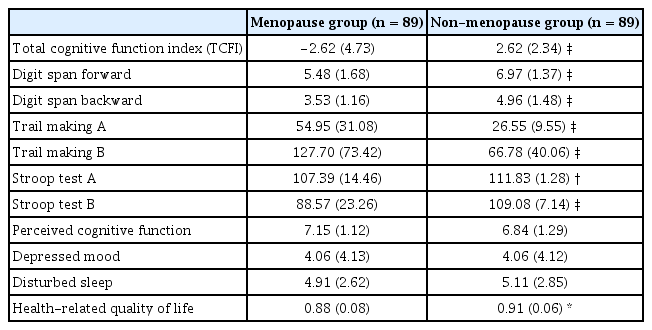
Menopausal women differed from non-menopausal women in their performance on neuropsychological tests (t = -9.37, p < 0.001) and HRQOL (t = −2.55, p = 0.012). Perceived cognitive function was not significantly different between groups (t = 1.75, p = 0.082). The severity and occurrence of depressed mood and disturbed sleep were similar for both groups (Table 2).
3. Correlations of HRQOL with cognition, sleep, and depression in menopausal and non-menopausal women
Correlation coefficients were computed to identify potential covariates that are independently associated with HRQOL. HRQOL was significantly associated with the TCFI score computed by neuropsychological test performance (r = 0.47, p < 0.001), depressed mood (r = −0.30, p < 0.01), and disturbed sleep (r = −0.32, p < 0.001) across all participants. The relationship between perceived cognitive function and HRQOL was not significant (p = 0.06).
When correlational analysis was conducted in each group, similar results to correlation coefficient analysis were observed for HRQOL, with higher correlational coefficients in the menopause group (range: −0.30 − 0.55) than in the non-menopause group (range: −0.28 – −0.40). The only difference in the relationships found between two groups was that a significant relationship between the TCFI score and HRQOL was observed in the menopause group, but not in the non-menopause group (p < 0.001; Table 3).
4. Factors associated with HRQOL
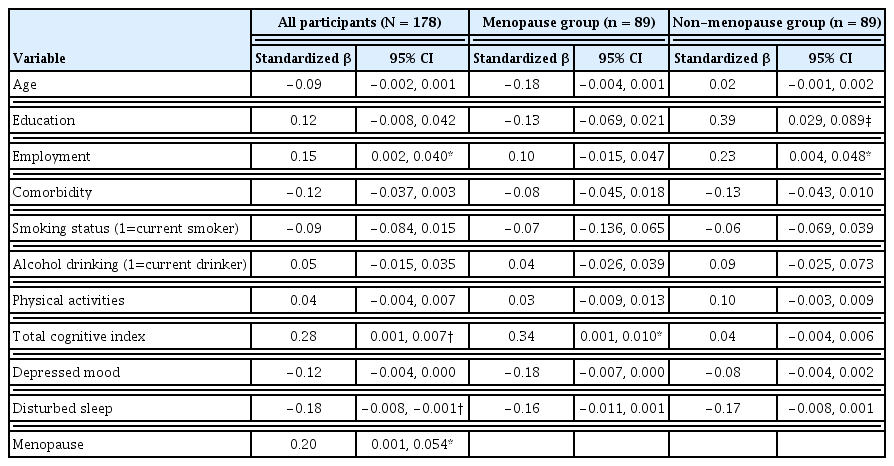
Multiple regression analyses were performed to examine the association between the menopause and HRQOL after controlling for potential covariates (age, education, employment, comorbidity, neuropsychological test performance, depressed mood, disturbed sleep), and to assess whether there was a difference in factors associated with HRQOL depending on menopausal status. For the regression model including all participants, the independent variables explained 38.5% of the variance in HRQOL (multiple R = 0.621, adjusted R2 = 0.385, F = 9.451, p < 0.001). Being menopausal (β = 0.20), employed (β = 0.15), having a better cognitive performance (β = 0.28), and lower levels of disturbed sleep (β = −0.18) were associated with a higher HRQOL. Interestingly, factors associated with HRQOL differed between menopause and non-menopause groups. Cognitive performance (β = 0.34) was solely associated with HRQOL in the menopause group, while socioeconomic variables such as education (β = 0.39) and employment status (β = 0.23) correlated with HRQOL in the non-menopause group (Table 4).
Discussion
The current study examined cognitive function and HRQOL in 2 groups of women categorized according to menopausal status to inform the development of interventions to maintain cognitive function, improve HRQOL, and support postmenopausal women through aging.
The findings in this study showed that menopausal women had lower scores on neuropsychological tests for attention, working memory, and executive function compared with non-menopausal women. It has been reported that estrogens play neuroprotective roles in preventing or delaying both normal cognitive decline and age-associated neurodegenerative diseases in the brain [37]. Despite controversies regarding the complex mechanisms of the neuroprotective actions of estrogen and the magnitude of the beneficial effect, it is well known that estrogen deprivation after natural or surgical menopause is significantly associated with the risk of Alzheimer’s disease in women [38]. In a recently published longitudinal study which observed cognitive changes in 2,124 middle-aged women whose median follow-up period was 6.5 years, processing speed was mainly linked with the negative effects of ovarian and chronological aging models, after controlling for practice effects and covariates such as sociodemographic factors, use of sex hormones, history of oophorectomy, comorbidity, and physical-psychological symptoms [6]. Specifically, a gradual decline in processing speed measured using the Symbol Digit Modalities test occurred in both the ovarian aging model with 0.28 unit decline per year and chronological model with 0.25 unit decline per year [6]. Given the shared effects of biological changes after the menopause and chronological aging on longitudinal declines in cognitive function, early interventions would be beneficial for reducing aging- and menopause-related cognitive decline in later life [39].
In this current study, menopausal women who had worse neuropsychological performance, experienced lower levels of HRQOL, while this association between cognitive performance and HRQOL did not occur in non-menopausal women. To our knowledge, this study is the first to identify whether there was a difference in the association between cognitive function (measured by standard neuropsychological tests on attention, working memory, and executive function) and HRQOL (measured by EQ-5D), according to menopausal status in women living in the community who were cognitively intact. This finding is consistent with the results of a community-based study showing a worse cognitive performance, and is reflective of menopausal women’s ability to perform everyday life activities leading to a reduced HRQOL [9]. Activities affected by cognitive decline encompass various aspects of life, from basic to instrumental activities of daily living, depending on the degree of cognitive dysfunction reached by the individual. Mild cognitive impairment is related to problems with activities requiring higher neuropsychological functioning, such as driving, managing medications and finances, keeping appointments, and using the telephone and technologies, while even basic activities, such as feeding, dressing, bathing, and grooming, are more likely to cause difficulties when symptoms of cognitive impairment get worse [40–42]. Taken together, the association between cognitive function and HRQOL may be stronger in menopausal women than in women who were non-menopausal, possibly because of the effect of menopause-induced estrogen deprivation. Future studies are needed to clarify the role of changes in the menopausal status through mediation and moderation analyses in this causal model.
There is compelling evidence to show that there is an association between estrogen and neurological structures, and its function in the hypothalamus, hippocampus, and neocortex of the female brain, which has multifaceted roles in cognitive function, especially working memory, processing speed, and executive function [28,43]. Given the potential effect of estrogen on individual domains of cognition, it is important to select domain-specific neuropsychological tests, rather than a global assessment of cognitive status to precisely determine the effect of cognitive function in cognitively intact people living in the community. It has been reported that HRQOL in older women was associated with executive function as measured by the Trail Making test, the Digit Span test, and the Timed Up and Go test, while global cognitive function as assessed by the Mini-Mental State Examination was not directly associated with HRQOL in high functioning women living in the community, regardless of age [10]. In this current study, standard neuropsychological tests (i.e., Trail Making, Digit Span, and the Stroop tests) were also administered to measure attention and working memory, which were required for executive processes in the brain. This finding revealed that performance on these neuropsychological tests may function methodologically as sensitive tools to examine goal-driven cognitive functions, which were known to be significant predictors of daily functioning in older adults [41].
Unlike objectively measured cognitive performance, perceived cognitive function did not have a significant association with HRQOL in both menopausal and non-menopausal women in this study. This finding was not consistent with the results of a study in older adults with subjective cognitive impairment that examined the subjective reports of cognitive decline and HRQOL [44]. This comprehensive study showed that the severity of subjective cognitive impairment was associated with reduced HRQOL despite the sample heterogeneity, methodological approach, and study design. However, this should be carefully interpreted because conceptualizations of subjective perception in cognitive function may vary depending on age groups. For example, young adults were likely to experience reduced cognitive function when experiencing difficulties in relationships with their friends, whereas older adults seemed to exhibit cognitive problems that affect their living, family, and social activities. The instrument used in this study is a well-developed and theory-driven tool to assess attention, working memory, and executive function, but it is difficult to exclude the possibility that some items in this instrument may not sufficiently capture age-specific complaints about subjective ineffectiveness in cognitive function. Thus, it may be necessary to develop a new instrument to assess age-specific cognitive complaints or invest scholarly effort into improving the selection of outcome measurement tools.
Depressed mood and disturbed sleep were not significantly related to HRQOL based on menopausal and non-menopausal status, while there was a significant association of disturbed sleep with HRQOL across all participants. One explanation for this finding is that the intensity of depressed mood and disturbed sleep may not have been great enough to affect HRQOL, given that study participants had mild levels of sleep and depression problems in both groups. However, clearly changes in mood and sleep patterns are strong predictors of HRQOL as well as health outcomes associated with the menopause. Thus, it is essential to establish a causal model to explain the direct and indirect paths between HRQOL and associated physical, emotional, and cognitive factors, and to investigate the role of menopause in the proposed model.
There are 2 limitations in this study. Firstly, this study did not provide longitudinal data to explain the changing pattern of cognitive function and HRQOL with the onset of the menopause transition. Secondly, the study sample did not have men as a comparison group; thus, future studies are needed to examine whether there is a difference in the impact of cognitive decline in addition to the menopause, on HRQOL between men and women aged 50 or older. Regardless of these limitations, this study provides new evidence of the impact of cognitive function after menopause on HRQOL and the importance of theoretically congruent neuropsychological assessment among cognitively intact women living in the community.
Conclusion
The current study examined cognitive function and HRQOL in 2 groups of women categorized according to menopausal status, to clarify the findings of prior research and to inform the development of interventions to maintain cognitive function, improve HRQOL, and support menopausal women during aging. Cognitive function, especially attention and working memory, was observed as an important factor associated with HRQOL in menopausal women. Non-menopausal women did not show this significant association. This result suggests the potential value of menopausal status and cognition as important factors of HRQOL. Healthcare providers should pay more attention to the relationship between cognitive decline, menopause, and HRQOL when determining target groups and developing programs to improve daily functioning, life satisfaction, and HRQOL during aging.
Acknowledgments
This study was funded by the Chungnam National University (no.: 2016-1794-01).
Notes
Conflicts of Interest
The authors have no conflicts of interest to declare.