Treatment with Sofosbuvir and Daclatasvir (with or without Ribavirin) Improves Patient Reported Outcomes in Hepatitis C
Article information
Abstract
Objectives
To evaluate the impact of 3 treatment regimens upon health-related quality of life and work productivity using patient-reported outcomes (PROs) in chronic hepatitis C infected patients: sofosbuvir (SOF) + daclatasvir (DCV); SOF + DCV + ribavirin (RBV); SOF + simeprevir (SMV).
Methods
4 questionnaires were used to evaluate PROs before, during and after treatment: Short Form-36 (SF-36), Chronic Liver Disease Questionnaire (CLDQ) - hepatitis C virus (HCV), Work Productivity and Activity Index, Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F).
Results
Of the global sample of 55 patients included in this study; SOF + DCV (n = 10); SOF + DCV + RBV (n = 29); SOF + SMV (n = 16) all had a statistically significant improvement in SF-36, CLDQ and FACIT-F scores during and post-treatment. No statistically significant differences in the PRO questionnaire values were observed between the distinct treatment regimens. The SOF and SMV patient groups presented higher mean PRO variations during and post-treatment, compared to the other groups: SF-36 functional capacity (16.1); SF-36 mental health (21.4); CLDQ activity (1.8); CLDQ emotional function (1.2); FACIT-F physical well-being (8.0); Total FACIT-F (21.6).
Conclusion
Treatment with SOF + DCV, with or without RBV, results in an improved PRO similar to treatment with SOF + SMV in chronic hepatitis C patients.
Introduction
Hepatitis C virus (HCV) infects around 2.8% of the world’s population, with estimates of 3 to 4 million new infections per year around the world [1, 2]. Chronic hepatitis C (CHC) develops in 10% to 20% of cases leading to liver cirrhosis, and 1% to 3% of those infected can develop hepatocellular carcinoma [3–5].
The concept of quality of life (QOL) represents the patient’s perception of the effect of the disease and the treatment given, upon physical, psychological and social aspects of their life [6]. Patient-reported outcomes (PROs) are measures exclusively reported by the patients themselves, without the influence of the interviewer, on issues that include health-related quality of life (HRQOL) and work productivity (WP) [7].
Patients infected with HCV present with a compromised HRQOL [8–10] and WP, with deteriorating rates of both absenteeism and presenteeism [11]. Treatment with interferon (IFN) is available to these patients but has been associated with limited therapeutic success rates and a high presence of side effects, including depression and fatigue [12, 13].
The era of new direct-acting antivirals (DAAs) has promoted positive changes in CHC care. IFN-free DAAs are highly effective at viral eradication, whilst also improving patient well-being during and post-treatment, as observed using HRQOL and WP questionnaires [14, 15].
To the best of our knowledge, no study evaluating PROs in HCV-infected patients treated with daclatasvir (DCV) in combination with sofosbuvir (SOF), with or without ribavirin (RBV), has been conducted. The aim of our study was therefore, to use PROs to compare the impacts of 3 different treatment regimens of: SOF and simeprevir (SMV); SOF and DCV and RBV; or SOF and DCV; on HRQOL and WP, before, during, and post-different treatment in a CHC patient sample.
Materials and Methods
From December 2015 to June 2016, the CHC cohort receiving IFN-free DAAs was evaluated under the following regimens: SOF 400 mg and SMV 150 mg/day; SOF 400 mg and DCV 60 mg/day; or SOF 400 mg and DCV 60 mg and RBV 1000 to 1200 mg/day; from 12 to 24 weeks, at the Liver Disease Outpatient Clinic of the Gastroenterology Department of the Gaffrée and Guinle University Hospital (HUGG), in Rio de Janeiro, Brazil.
The sample was not randomized or blinded to the outcome of sustained virological response (SVR). The patients participating in the study presented at a previous follow-up visit and adequate outpatient follow-up was maintained by the responsible physicians. Inclusion criteria for the study were patients with established HCV (genotypes 1, 2 or 3) who were either virgins or previously treated for HCV and aged 18 years or older. Exclusion criteria were as follows: co-infection with the hepatitis B virus; co-infection with human immunodeficiency virus (HIV); clinical examination in patient screening for decompensated liver disease (ascites, hepatic encephalopathy, active digestive bleeding) and the presence of hepatocellular carcinoma.
Clinical and socio-demographic data from patient medical records were used. The METAVIR score was based on transient hepatic elastography (Fibroscan), where scores were classified as follows: F0: without fibrosis; F1: portal fibrosis without septa; F2: some septa; F3: numerous septa without fibrosis; and F4: cirrhosis.
This study was conducted in accordance with the Declaration of Helsinki (1964). The research protocol was approved by the HUGG Research Ethics Committee, in accordance with the Brazil Platform (CAAE 47784015700005258). All patients signed an informed consent form.
For the PRO evaluations, 4 widely used, self-administered questionnaires that were validated for CHC patients were applied [16], either before or after their clinical consultations. In patients undergoing a 12-week treatment regimen, the evaluation was performed before the beginning of the treatment, after treatment began (at Week 4 and at Week 12), and 4 weeks post-treatment. In patients undergoing a 24-week treatment regimen, the evaluation was performed before the beginning of the treatment, after treatment began (at Weeks 4, 12, and 24), and 4 weeks post-treatment.
The SF-36 (Medical Outcomes Study 36 – Item Short-Form Health Survey) - RAND-36 - is a multi-dimensional HRQOL questionnaire, formed by 36 items distributed into 8 scales or components, namely, functional capacity, physical aspects, pain, general health, vitality, social aspects, emotional aspects and mental health. It was translated and cross-culturally adapted and validated in Brazil [17–19].
The CLDQ-HCV (Chronic Liver Disease Questionnaire) was translated, adapted and validated in Brazil, and presents 29 separate questions comprising 6 domains: abdominal symptoms, fatigue, systemic symptoms, activity, emotional function and worry [20–22].
The Work Productivity and Activity Impairment (WPAI) is a questionnaire that evaluates the effects of health on WP, as well as productivity outside the work environment. It consists of 6 questions: current employment or unemployment situation; the number of hours not worked due to health problems and other reasons; the number of hours actually worked; how much the patient’s health problems have affected WP; and how much the patient’s health problems have affected daily activities. The Brazilian Portuguese version has been proven valid and reliable for measuring WP [23].
The FACIT (Functional Assessment of Chronic Illness Therapy) is a fatigue questionnaire validated in Brazil and licensed by facit.org. It comprises 40 items distributed in the physical, emotional, social and functional wellbeing spheres, as well as sub-scale fatigue spheres [24–26].
The descriptive statistics for the measuring scales obtained from the questionnaires were calculated, and appropriate tables and graphs were constructed. The social, demographic and clinical variables were analyzed by Fisher’s Exact Test. The behavior of the scales throughout the treatment was analyzed by the Friedman test and the comparative analysis of the scale variations according to treatment was performed by the Kruskall-Wallis test. The statistical package SP v.24, was used for all statistical analyses.
Results
1. Demographics and patient characteristics
Analysis was performed on 55 patients included in the study. The socio-demographic characteristics of the sample indicated that 30 patients were older than 60 (range 42–84 years), most of them female (n = 38), and that 32 patients were defined as white and 35 lived with a partner. The time to disease diagnosis was more than 10 years in 24 patients, with genotype 1 being the most frequent (n = 49) followed by genotype type 2 (n = 6).
METAVIR 3 or 4 fibrosis was observed in 47 patients who underwent the following treatment regimens: SOF + DCV (n = 10); SOF + DCV + RBV (n = 29); SOF + SMV (n = 8). Thirty patients previously received IFN treatment that was not effective. The treatment time with DAAs was 12 weeks in 48 patients, and 24 weeks in 7 patients (SOF + DCV, n = 1; SOF + DCV + RBV, n = 6). Fisher’s exact test indicated even distribution within and between the treatment subgroups was homogeneous for social, demographic and clinical variables. The most prevalent transmission route in the total study sample was a history of blood transfusion (n = 39), followed by a needlestick injury (n = 9), indicating these are risk factors for contracting HCV.
Therapeutic regimens were used without randomization and the patients were not blinded to the type of treatment or to the scientific rationale of SVR, with 14 patients undergoing treatment with SOF + SMV, 30 patients undergoing treatment with SOF + DCV + RBV, and 11 patients undergoing treatment with SOF + DCV (Table 1). SVR at the end of treatment was achieved by 54 patients.
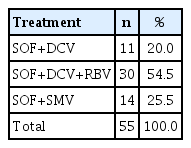
Patients with hepatitis C treated HUGG, between December 2015 and June 2016, according to treatment regimen, in Rio de Janeiro, Brazil.
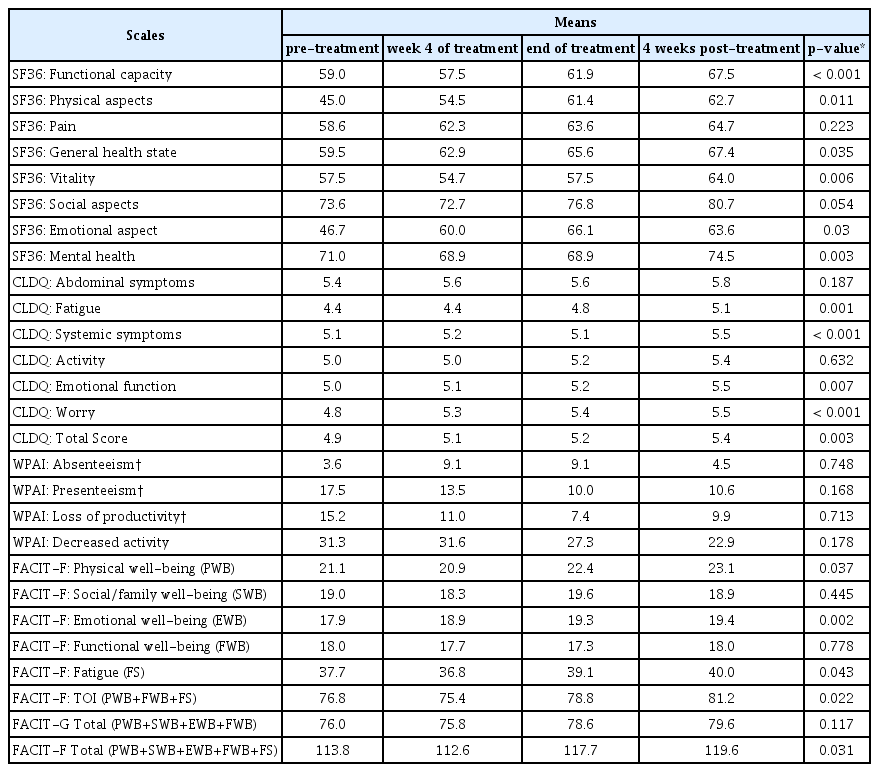
The comparisons of the different treatment regimens in relation to PRO behavior scales throughout the treatments were obtained first by the prior determination of the normality distribution of the means of the scale components by the Kolmogorov-Smirnov test. Thus, subsequent scale-behavior trends were obtained by non-parametric analyses (Table 2). Statistically increased means were observed during and post-treatment for the 3 treatment regimens for most SF-36, CLDQ, and FACIT-F components.
2. Comparison of pre-treatment SF36, CLDQ, WPAI and FACIT-F scores
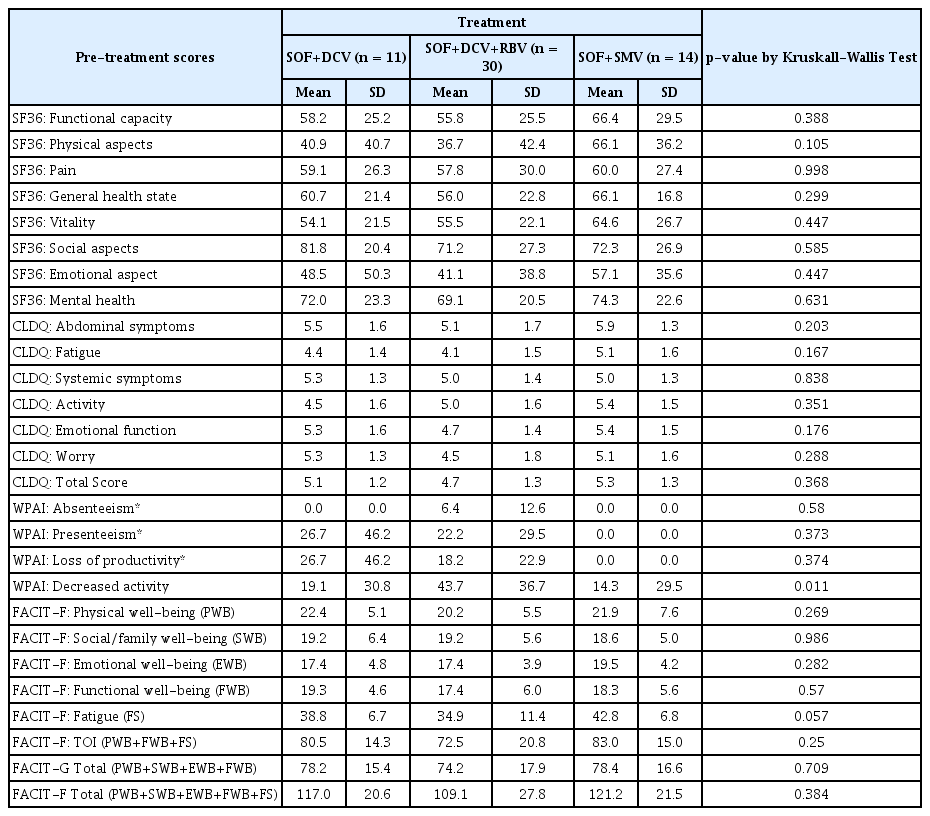
Descriptive statistics of the pre-treatment scores revealed that the type of treatment to which the patients were subjected to did not significantly influence the initial means of the scale components, indicating a probable uniformity between the groups in the pre-treatment stage (Table 3).
3. Comparison of SF36, CLDQ, WPAI and FACIT-F scores from pre-treatment through to 4 weeks post end of treatment
In a global view of the scales, trends for positive variation in all 4 questionnaires were observed at Week 4 (Table 4), at the end of treatment (Table 5) and, mainly, at 4 weeks post-treatment (Table 6).
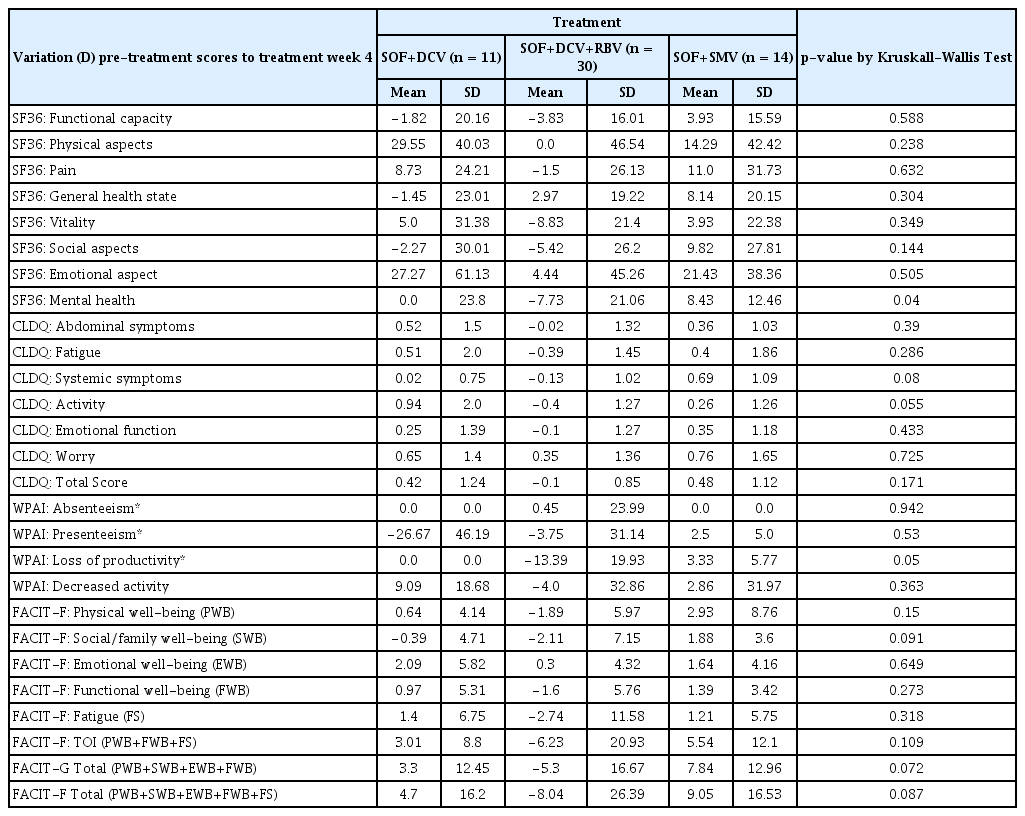
Descriptive statistics of scores variation (D) from pre-treatment to treatment week 4 of the SF36, CLDQ, WPAI e FACIT-F in 55 hepatitis C patients, by treatment regimens.
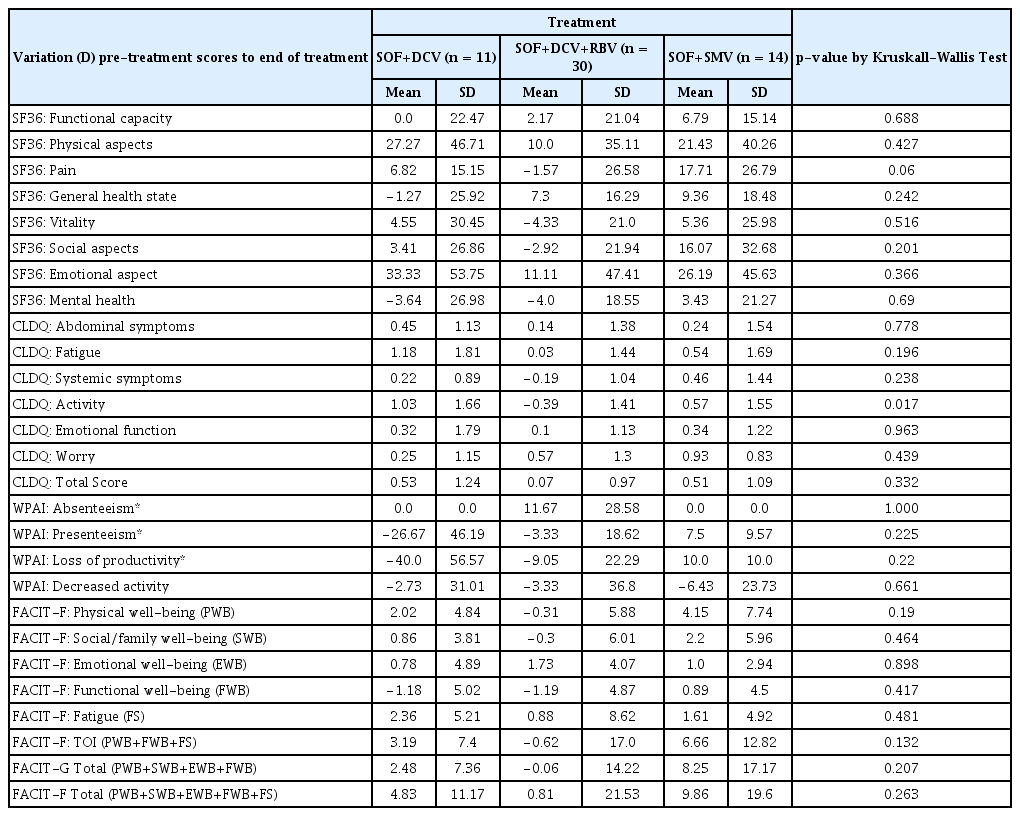
Descriptive statistics of scores variation (D) from pre-treatment to end of treatment of the SF36, CLDQ, WPAI e FACIT-F in 55 hepatitis C patients, by treatment regimens.
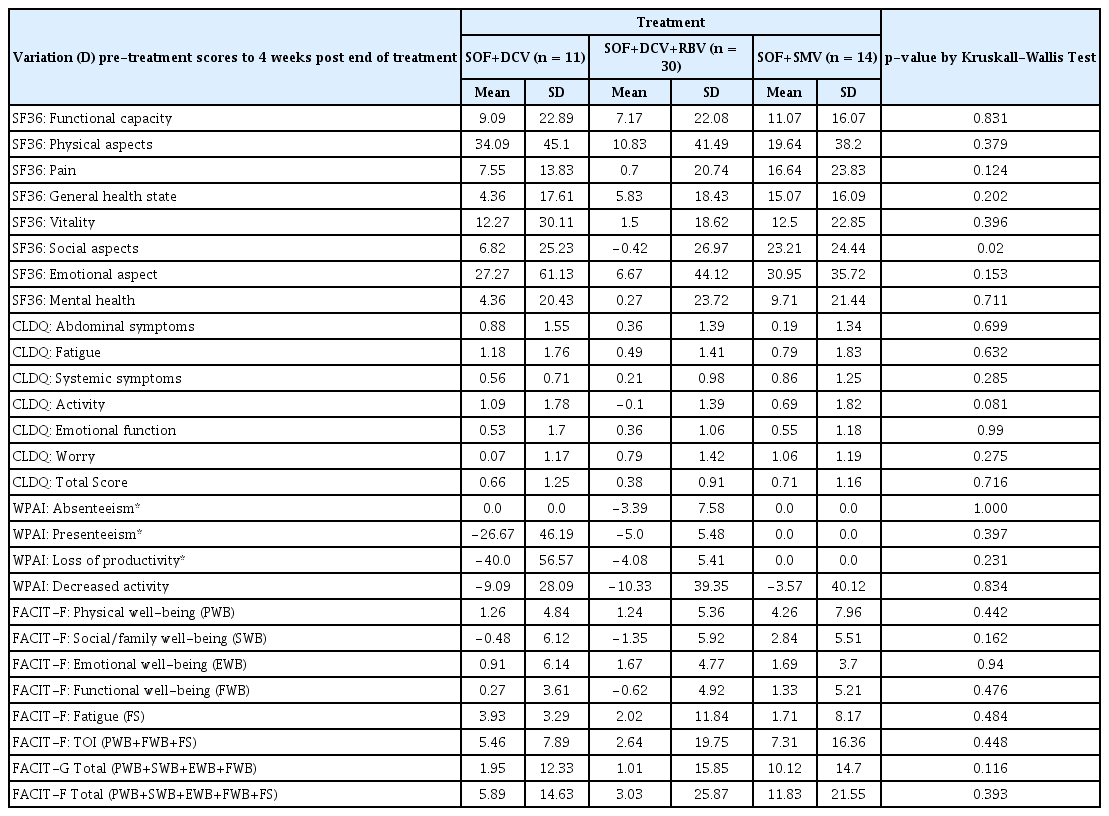
Descriptive statistics of scores variation (D) from pre-treatment to 4 weeks post end of treatment of the SF36, CLDQ, WPAI e FACIT-F in 55 hepatitis C patients, by treatment regimens.
No statistically significant difference in scale behavior according to treatment regimen, was observed by the Kruskall-Walls test.
More frequent decreases were observed at Week 4 of the treatment (Table 4) compared to the other evaluated weeks, with a predominance of a slight decrease in the SOF+DCV+RBV group. These decreases occurred for SF 36 functional capacity (−3.8); SF-36 pain (−1.5); SF-36 vitality (−8.8); SF-36 emotional aspects (−5.4); SF-36 mental health (−7.7); CLDQ fatique (−0,4); CLDQ systemic symptoms (−0.1); CLDQ activity (−0.4); FACIT-F physicial well-being (−1.9); FACIT-F fatigue (−2.7) and Total FACIT-F (−8.0). The SOF + SMV treatment group showed better average scores regarding general perception of most of the scale components, compared with the SOF + DCV (without RBV) group.
In the final week of treatment, slight decreases of the means in some instrument components was observed, mainly in the SOF + DCV + RBV group. The absolute comparison of the mean values in the final week of the treatment regimen, indicated a recurrent increase in the SOF + SMV group compared to the SOF + DCV groups with and without RBV (Table 5).
The WPAI scale shows a trend of improvements in mean values throughout the treatment regimen (negative values in WPAI indicate WP improvement). However, only 16 patients stated that they were currently working, which generated a limited sample regarding productivity analysis, as well as instability in the maintenance of work activities over the course of the treatment, resulting in statistically non-significant results (Table 4, 5 and 6).
At Week 4 post-treatment (Table 6), the most positive amplitude variation was maintained in the SOF and SMV groups, namely for SF-36 functional capacity (16.1); SF-36 physical aspects (38,6); SF-36 vitality (22.9); SF-36 mental health (21.4); CLDQ fatigue (1.8); CLDQ activity (1.8); CLDQ emotional function (1,2); FACIT-F physical well-being (8.0); FACIT-F social/family well-being (5.5); FACIT-F fatigue (8.2) and Total FACIT-F (21.6).
Discussion
This current study was the first to comparatively evaluate the effect of different treatment regimens applying SOF + DCV, SOF+ DCV + RBV, and SOF + SMV, upon PRO in patients with CHC.
The high number of patients treated with SOF + DCV, or SOF + DCV + RBV was due to the greater accessibility from the Ministry of Health for this therapy. In this scenario, no controlled randomization was applied, since patients were awaiting treatment.. The random allocation of the participants did not influence the data, leading to heterogeneity of the initial means, indicating that the pre-treatment groups can be compared (Table 3).
The study was not blinded when the SVR result was applied. The patients were not informed of the results of the viral eradication response to treatment in case this influenced the assessment scores of their well-being, as reported in previous studies. [15].
In general, the IFN-free DAAs promoted improvements in PROs scores (SF-36, CLDQ and FACIT-F). This result is in line with earlier studies demonstrating the benefit of other DAA-based treatment regimens on PROs [15, 27].
The perception of a discrete decrease in the means at Week 4, predominantly in the DCV and RBV group, is in agreement with a study performed by Younossi et al. [28]. Younossi et al. observed decreases in PROs of −7.0% in the group undergoing SOF and RBV treatment, as opposed to an increase of +11.6% in the group undergoing ledipasvir and SOF treatment (p < 0.0001), which, in a multivariate analysis, represented an independent association with a −9.0% decrease in PROs in the SOF and RBV group [28]. The decrease in the means of the RBV group of our study may be justified by the possible unwanted side effects of this drug, such as anemia. However, the negative effect for RBV was not statistically significant.
The only previous study on the influence of DCV on PROs was a retrospective analysis of 33 patients co-infected with HIV. HIV co-infection may adversely affect the interpretation of the HRQOL deterioration [29]. In this study, SOF + DCV, or DCV and ledipasvir regimens were better tolerated and presented improved scores for SF-36 physical health (41.4 ± 9.7) and fatigue (37.8 ± 14.0) compared to the IFN-based regimen. Our study observed a consistent trend towards an improvement in patients treated with SOF + SMV, compared to the SOF + DCV and SOF + DCV + RBV groups.
The absolute difference of the means with regard to the pre-treatment visit is more significant at Week 4 after the end of treatment, especially in the SOF + SMV groups. Despite the observed absolute differences of the means, the magnitudes of these differences were not statistically significant. It is possible that extending the evaluation to 12 weeks post-treatment could allow more time for a more significant positive effect on PROs to be observed.
The WPAI scores showed little responsiveness in detecting WP in our sample. We understand that the number of patients actually working and the informality at work that causes oscillation in these data is a reflection of the current socioeconomic situation in Brazil. Other researchers have found similar obstacles [30].
Although the size of our patient population helped in understanding the impact of the new treatments on the HRQOL in HCV infected patients, a greater number of patients is required for multivariate analyses.
In summary, the results from this study demonstrated that CHC patient treatment with DCV, in combination with SOF, with or without RBV, causes an impact in PROs similar to treatment with SOF in combination with SMV. The comparison between the groups (SOF + SMV, SOF + DCV, SOF + DCV + RBV) indicated no statistically significant difference in HRQOL and PT scores either during treatment or post-treatment. Studies with larger patient numbers are needed to fully understand the effects of DCV and RBV in PROs in other populations presenting CHC.
Notes
Conflicts of Interest
The authors declare that they have no conflicts of interest.