Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 13(5); 2022 > Article
-
Brief Report
Adverse events of the Pfizer-BioNTech COVID-19 vaccine in Korean children and adolescents aged 5 to 17 years -
Seontae Kim1
 , Yeseul Heo1
, Yeseul Heo1 , Soon-Young Seo1
, Soon-Young Seo1 , Do Sang Lim1
, Do Sang Lim1 , Enhi Cho2
, Enhi Cho2 , Yeon-Kyeng Lee1
, Yeon-Kyeng Lee1
-
Osong Public Health and Research Perspectives 2022;13(5):382-390.
DOI: https://doi.org/10.24171/j.phrp.2022.0233
Published online: October 14, 2022
1Adverse Event Management Team, Immunization Safety Group, COVID-19 Vaccination Task Force, Korea Disease Control and Prevention Agency, Cheongju, Korea
2Immunization Safety Group, COVID-19 Vaccination Task Force, Korea Disease Control and Prevention Agency, Cheongju, Korea
- Corresponding author: Yeon-Kyeng Lee Adverse Event Management Team, Immunization Safety Group, COVID-19 Vaccination Task Force, Korea Disease Control and Prevention Agency, 187 Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju 28159, Korea E-mail: yeonkyenglee@cdc.go.kr
© 2022 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- This study aimed to identify potential safety signals and adverse events following the primary Pfizer-BioNTech coronavirus disease 2019 (COVID-19) vaccination series among children and adolescents aged 5 to 17 years in the Republic of Korea.
-
Methods
- Adverse events reported through the COVID-19 vaccination management system (CVMS, a web-based passive vaccine safety surveillance system) and adverse events and health conditions collected from a text message-based survey were analyzed.
-
Results
- A total of 14,786 adverse events among 5 to 17-year-old children and adolescents were reported in the CVMS; 14,334 (96.9%) were non-serious and 452 (3.1%) were serious, including 125 suspected cases of acute cardiovascular injury and 101 suspected cases of anaphylaxis. The overall reporting rate was lower in 5 to 11-year-old children (64.5 per 100,000 doses) than in 12 to 17-year-old adolescents (300.5 per 100,000 doses). The text message survey identified that local and systemic adverse events after either dose were reported less frequently in 5 to 11-year-old children than in 12 to 17-year-old adolescents (p<0.001). The most commonly reported adverse events were pain at the injection site, myalgia, headache, and fatigue/tiredness.
-
Conclusion
- The overall results are consistent with the results of controlled trials; serious adverse events were extremely rare among 5 to 17-year-old children and adolescents following Pfizer-BioNTech COVID-19 vaccination. Adverse events were less frequent in children aged 5 to 11 years than in adolescents aged 12 to 17 years.
- In the Republic of Korea (ROK), only the Pfizer-BioNTech (BNT-162b2) messenger RNA (mRNA) coronavirus disease 2019 (COVID-19) vaccine has been authorized for use in persons aged ≥5 years based on safety and efficacy data from controlled trials organized in the United States (US) by the Korea Ministry of Food and Drug Safety [1–3]. The Pfizer-BioNTech COVID-19 vaccine (30 µg, 0.3 mL each) was initially authorized for use in persons aged ≥16 years on March 5, 2021 [4], and expanded to include adolescents aged ≥12 years on July 16, 2021 [5]. The use of the Pfizer-BioNTech COVID-19 vaccine (10 µg, 0.2 mL each) for children aged 5 to 11 years was authorized on February 23, 2022 [6].
- Since then, the Pfizer-BioNTech vaccine has been nationally distributed to adolescents aged 16 to 17 years starting on October 18, 2021, and to adolescents aged 12 to 15 years starting on November 1, 2021, following a decision by the Korea Advisory Committee on Immunization Practices (KACIP) in 2021 [7,8]. Following the KACIP in 2022, the Pfizer-BioNTech vaccine was offered to children aged 5 to 11 years starting on March 31, 2022, with the recommendation of an 8-week interval between the 2 doses based on findings that showed increased safety and efficacy with the extended interval [9–12].
- To monitor adverse events following immunization (AEFIs) and identify potential safety signals for further evaluation, the Korea Disease Control and Prevention Agency (KDCA) manages the COVID-19 vaccination management system (CVMS, a web-based passive vaccine safety surveillance system), in which doctors and forensic pathologists can report AEFIs regardless of a causal association between events and vaccines as per the Infectious Disease Control and Prevention Act [13]. The KDCA also operates a text message-based vaccine safety surveillance system that surveys adverse events and health conditions following COVID-19 vaccination for particular populations who consent to receive text message surveys through smartphones on the day of their first vaccination [13].
- This study aimed to identify potential safety signals and adverse events following the primary series of Pfizer-BioNTech vaccination, including dose 1 and dose 2, for children and adolescents aged 5 to 17 years in the ROK. This study analyzed data on adverse events reported in the CVMS and the text message-based vaccine safety surveillance system.
Introduction
- COVID-19 Vaccination Management System
- From March 5, 2021 to July 2, 2022, in total, 4,995,280 primary doses of the Pfizer-BioNTech vaccination series were administered to children and adolescents aged 5 to 17 years in the ROK, and 14,786 adverse events after vaccination were reported to the CVMS. Data on an additional dose (dose 3), vaccines other than the Pfizer-BioNTech vaccine for children aged 5 to 11 years (10 µg) and adolescents aged 12 to 17 years (30 µg), and vaccination that occurred abroad and before authorization for use in children and adolescents in the ROK were excluded. Adverse events reported in the CVMS were divided into non-serious and serious events in accordance with the Guidelines for Adverse Events Following COVID-19 Immunization [13]. Non-serious events included common symptoms such as redness, pain, and swelling at the injection site, myalgia, fever, headache, chills, and others. The following adverse events were classified as serious: death, anaphylaxis, adverse events of special interest (AESIs), intensive care unit admission, life-threatening events, permanent disability or sequelae, and others. The characteristics of adverse events reported in the CVMS among 5 to 17-year-old children and adolescents were analyzed by sex, age group, and vaccine dose. The types of symptoms and signs were presented in descending order of the number of cases reported as adverse events. The events do not indicate medically confirmed diagnoses, as adverse events reported to the CVMS are suspected cases.
- Text Message-Based Vaccine Safety Surveillance System
- Text messages were sent to parents or guardians of children and adolescents aged 5 to 17 years in the ROK who received the primary series of the Pfizer-BioNTech vaccine, on a daily basis until day 7 post-vaccination to investigate adverse events and health conditions. A total of 10,398 adolescents aged 12 to 17 years from December 13, 2021 to January 26, 2022, and 1,025 children aged 5 to 11 years from March 31 to June 20, 2022, were enrolled in the text message-based surveillance system. The surveys asked questions about experiences of local and systemic adverse events, limits to normal daily activities, and visits to medical facilities following vaccination. The respondents were able to report multiple adverse events on each day. The characteristics of respondents were described by sex and age, and adverse events and health conditions reported at least once during days 0¬ to 7 following vaccination were assessed by vaccine doses and age groups. All variables were examined using the chi-square or Fisher exact test as appropriate to compare adverse events and health conditions between age groups and vaccine doses. A p-value <0.05 indicated statistical significance.
- SAS ver. 9.4 (SAS Institute, Cary, NC, USA) was used to conduct all analyses. The passive surveillance activity was conducted and authorized by the KDCA; the study was not subject to institutional review board approval under government regulations. The study of the text message-based surveillance was exempted from review by the Public Institutional Review Board designated by the Korea Ministry of Health and Welfare (No: P01-202206-01-033).
Materials and Methods
- Adverse Events Reported in the COVID-19 Vaccination Management System
- From March 5, 2021 to July 2, 2022, the CVMS confirmed a total of 14,786 adverse events among children and adolescents aged 5 to 17 years after primary doses of the Pfizer-BioNTech vaccination series (Table 1); 14,334 (96.9%) were non-serious and 452 (3.1%) were serious. Serious adverse events included death (5, 0.0%), suspected anaphylaxis (101, 0.7%) and major adverse events including AESIs for COVID-19 vaccines (346, 2.3%). During the study period, 4,995,280 doses were administered to children and adolescents aged 5 to 17 years, and the overall reporting rate per 100,000 doses administered was 296.0 (dose 1, 270.0; dose 2, 323.3). The reporting rate per 100,000 doses after the primary vaccination series by sex was 283.6 in males and 309.2 in females. The reporting rate per 100,000 doses was lower in children aged 5 to 11 years (64.5/100,000 doses) than in adolescents aged 12 to 17 years (300.5/100,000 doses). Among non-serious adverse events, the most commonly reported symptoms based on the reporting rate per 100,000 doses were headache (75.4/100,000 doses), chest pain (68.4/100,000 doses), myalgia (43.1/100,000 doses), dizziness (41.3/100,000 doses), and nausea (36.9/100,000 doses) (Table 2). Among serious adverse events, acute cardiovascular injury including myocarditis/pericarditis (2.5/100,000 doses) had the highest reporting rate per 100,000 doses, followed by anaphylaxis, including anaphylactoid reactions (2.0/100,000 doses), convulsions or seizures (1.0/100,000 doses), acute paralysis (0.8/100,000 doses), and vaccine-associated enhanced disease (0.8/100,000 doses).
- Adverse Events Collected in the Text Message-Based Vaccine Safety Surveillance System
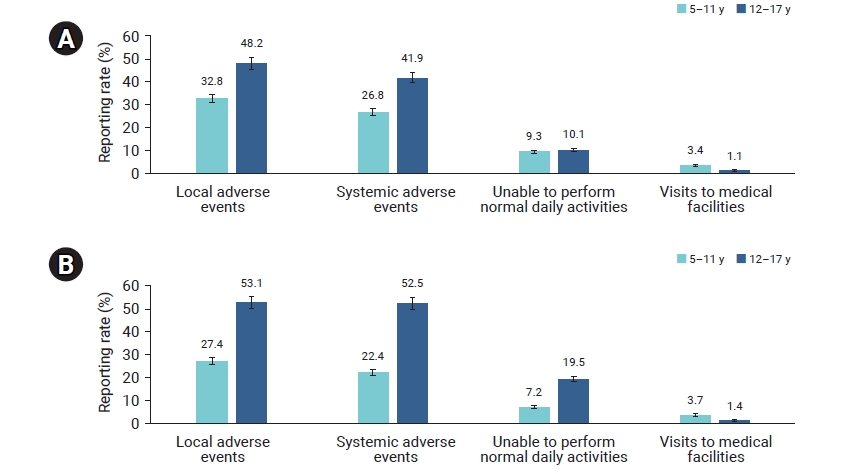
- From December 13, 2021 to June 20, 2022, the total number of children and adolescents aged 5 to 17 years enrolled in at least 1 text message survey on days 0 to 7 following Pfizer-BioNTech COVID-19 vaccination was 11,414 after dose 1 (male, 47.0%; female, 53.0%), and 3,688 after dose 2 (male, 46.8%; female, 53.2%) (Table 3). The number of respondents by age group was 1,025 (9.0%) after dose 1 and 541 (14.7%) after dose 2 among 5 to 11-year-old children, and 10,389 (91.0%) after dose 1 and 3,147 (85.3%) after dose 2 among 12 to 17-year-old adolescents respectively. During the week after either dose, local adverse events were reported more frequently than systemic adverse events in both age groups. The reporting rate of local adverse events after dose 1 was 32.8% in 5 to 11-year-old children and 48.2% in 12 to 17-year-old adolescents (p<0.001) (Figure 1; Table 4). After dose 2, the reporting rate of local adverse events was 27.4% in 5 to 11-year-old children and 53.1% in 12 to 17-year-old adolescents (p<0.001). For systemic adverse events after dose 1, 26.8% of 5 to 11-year-old children and 41.9% of 12 to 17-year-old adolescents responded (p<0.001), and the reporting rate after dose 2 was 22.4% for 5 to 11-year-old children and 52.5% for 12 to 17-year-old adolescents (p<0.001). The most frequently reported local adverse events were pain at the injection site and swelling, and the most commonly reported systemic adverse events were myalgia, headache, and fatigue or tiredness among both age groups after either dose (Table 4). Symptoms were most frequently reported during days 0 to 1, but were least frequently reported or disappeared during days 6 to 7 post-vaccination. Almost one-tenth of 5 to 17-year-old children and adolescents responded that they were unable to perform their normal daily activities after dose 1 (p=0.384), and this percentage after dose 2 was 7.2% in 5 to 11-year-old children and 19.5% in 12 to 17-year-old adolescents (p<0.001) (Figure 1; Table 4). Approximately 1.1% to 3.7% of 5 to 17-year-old children and adolescents visited medical facilities during days 0 to 7 after either dose. In addition, in the 5 to 11-year-old group, none of the local and adverse events showed statistically significant differences between the 2 doses except for pain (p=0.02) and myalgia (p=0.018), while among 12 to 17-year-old group, all dose 1 and dose 2 comparisons were statistically significant except for urticaria, diarrhea, and rash (Table S1).
Results
- Regarding the adverse events reported in the CVMS among children and adolescents aged 5 to 17 years after Pfizer-BioNTech COVID-19 vaccination, 96.9% were non-serious and 3.1% were serious. These proportions are similar to those in the safety data from the Vaccine Adverse Event Reporting System in the US; the great majority of adverse events were non-serious (5 to 11-year-old group, 97.4%; 12 to 17-year-old group, 90.7%), and serious adverse events were rare (5 to 11-year-old group, 2.6%; 12 to 17-year-old group, 9.3%) [14,15].
- The serious adverse events reported in the CVMS included 125 suspected cases of acute cardiovascular injury, 101 suspected cases of anaphylaxis, and 5 deaths. Reviewing 101 suspected cases of anaphylaxis, the number of cases was 1 in 5 to 11-year-old children (1.0%) and 100 in 12 to 17-year-old adolescents (99.0%). The number of cases was greater after dose 1 (82, 81.2%) than after dose 2 (19, 18.8%), but similar between males (46, 45.5%) and females (55, 54.5%). Other studies also found that anaphylaxis cases were reported more frequently after dose 1 than after dose 2, highlighting the significance of closely monitoring people who receive a first dose of the COVID-19 vaccine [16–18]. Furthermore, among 125 suspected cases of acute cardiovascular injury, the number of suspected myocarditis/pericarditis reports was 103; 1 case (1.0%) was in a 5 to 11-year-old, and 102 cases (99.0%) were in 12 to 17-year-old adolescents. Similar to previous findings [19–22], these cases were more frequent after dose 2 (67, 65.0%) than after dose 1 (36, 35.0%), and in males (79, 76.7%) than in females (24, 23.3%). However, since all adverse events reported in the CVMS are suspected cases, these events do not indicate medically confirmed diagnoses; therefore, a follow-up study will be required to medically confirm major suspected cases of serious adverse events for further evaluation, such as causality assessment between events and vaccines. Until now, none of the death reports has been assessed to be associated with vaccination based on medical records and epidemiological investigation results through an initial review conducted by provincial rapid response teams.
- The highest risk of myocarditis/pericarditis was observed in males aged 18 to 25 after dose 2 of the mRNA COVID-19 vaccine [19], and the reporting rate for mRNA-based COVID-19 vaccine-associated myocarditis appeared highest among males aged 12 to 29 years [20]. However, myocarditis/pericarditis cases following mRNA COVID-19 vaccination are rare among adolescents, and patients can recover quickly if treated well [23]. One study found no increased incidence of myocarditis/pericarditis after COVID-19 vaccination compared to other standard immunizations such as smallpox and influenza vaccines [22]. Furthermore, verified cases of myocarditis were rare among children aged 5 to 11 years after Pfizer-BioNTech vaccination in the US vaccine surveillance systems [24,25], and no cases of myocarditis were reported among 3,082 trial participants of the same age with 0 to 7 days of follow-up after dose 2 [26]. In this respect, the benefits of COVID-19 vaccination for children and adolescents aged 5 to 17 years are considered to outweigh the known and potential risks [27–30]; thus, this study does not support actions to exclude 5 to 17-year-old children and adolescents from vaccination and recommend that adverse events after COVID-19 vaccination should continue to be closely monitored to respond and provide additional information on COVID-19 vaccine safety, considering the limited information available in early safety monitoring [31].
- The results of the text message survey on adverse events and health conditions for children and adolescents aged 5 to 17 years following the primary Pfizer-BioNTech COVID-19 vaccination series are similar to the safety data reported in the v-safe system [14,15,24] and controlled trials [1,2] among those in the US; local adverse events were more common than systemic adverse events following either dose, and the majority of symptoms were mild, without major safety issues, and disappeared within a few days after vaccination. Moreover, injection site pain was the most common local adverse event, and fatigue, headache, and myalgia were the most common systemic adverse events in v-safe and controlled trials.
- According to the v-safe data [14,15], local (dose 1, 54.9%; dose 2, 56.8%) and systemic adverse events (dose 1, 35.3%; dose 2, 41.0%) among 5 to 11-year-old children were less frequently reported than local (dose 1, 62.7% to 63.9%; dose 2, 62.4% to 64.4%) and systemic adverse events (dose 1, 48.9% to 55.7%; dose 2, 63.4% to 69.9%) among 12 to 17-year-old adolescents. This trend is consistent with the results of the text message survey in this study; local and systemic adverse events were lower in children aged 5 to 11 years than in adolescents aged 12 to 17 years. However, this might be affected by the difference in the dose administered between children (10 µg) and adolescents (30 µg) [14] and the number of respondents enrolled in the surveys; thus, these figures should be compared with caution.
- This study has some limitations. First, the data were based on suspected adverse events following COVID-19 vaccination, and the events were not medically confirmed for an accurate diagnosis; thus, the results do not indicate causality. Second, as adverse events reported to the CVMS are based on individuals who visit medical facilities, the reports are subject to underreporting. Third, since text message surveys merely relied on self-reported responses, the number of adverse events reported might have been overestimated due to the likelihood of responding by parents or guardians. Fourth, as the text messages were sent during a particular period, the findings cannot be generalized to the entire child and adolescent population in the ROK. Nevertheless, the key strength of this study, as far as we know, is that this is the first study on COVID-19 vaccine safety among children and adolescents aged 5 to 17 years in real-world settings in the ROK based on national vaccine safety surveillance data. This study found consistent safety information on the Pfizer-BioNTech COVID-19 vaccine with controlled trials; serious adverse events following vaccination were extremely rare, with no major safety issues among children and adolescents aged 5 to 17 years.
Discussion
-
Ethics Approval
The passive surveillance activity was conducted and authorized by the public health authority; the study was not subject to the institutional review board approval under government regulations. The study of text message-based surveillance was exempted from review by the Public Institutional Review Board designated by the Korea Ministry of Health and Welfare (No: P01-202206-01-033).
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The data used in this study are protected under the Personal Information Protection Act.
-
Authors’ Contributions
Conceptualization: SK, SYS, YKL, EC; Data curation: SK, YH, DSL; Formal analysis: SK, YH, DSL; Investigation: SK, YH; Methodology: all authors; Validation: SK, SYS, YKL; Visualization: SK, YH; Writing–original draft: SK, YKL; Writing–review & editing: all authors.
Article information
-
Acknowledgements
- We thank the relevant ministries, including the Ministry of Interior and Safety, cities and provinces, and medical staff in health centers and medical facilities for their efforts in responding to the vaccine safety surveillance.
Supplementary Material
Table S1.

| Variable | No. of doses administered |
Adverse events (n=14,786)a) |
|||||
|---|---|---|---|---|---|---|---|
| Total | Non-serious adverse eventsb) |
Serious adverse eventsc) |
|||||
| Sub-total | Death | Anaphylaxis | Othersd) | ||||
| Total | 4,995,280 | 14,786 (296.0) | 14,334 (287.0) | 452 (9.0) | 5 (0.1) | 101 (2.0) | 346 (6.9) |
| Dose 1 | 2,555,595 | 6,899 (270.0) | 6,659 (260.6) | 240 (9.4) | 0 | 82 (3.2) | 158 (6.2) |
| Dose 2 | 2,439,685 | 7,887 (323.3) | 7,675 (314.6) | 212 (8.7) | 5 (0.2) | 19 (0.8) | 188 (7.7) |
| Sex | |||||||
| Male | 2,568,739 | 7,284 (283.6) | 7,028 (273.6) | 256 (10.0) | 3 (0.1) | 46 (1.8) | 207 (8.1) |
| Dose 1 | 1,314,670 | 3,329 (253.2) | 3,199 (243.3) | 130 (9.9) | 0 | 36 (2.7) | 94 (7.2) |
| Dose 2 | 1,254,069 | 3,955 (315.4) | 3,829 (305.3) | 126 (10.0) | 3 (0.2) | 10 (0.8) | 113 (9.0) |
| Female | 2,426,541 | 7,502 (309.2) | 7,306 (301.1) | 196 (8.1) | 2 (0.1) | 55 (2.3) | 139 (5.7) |
| Dose 1 | 1,240,925 | 3,570 (287.7) | 3,460 (278.8) | 110 (8.9) | 0 | 46 (3.7) | 64 (5.2) |
| Dose 2 | 1,185,616 | 3,932 (331.6) | 3,846 (324.4) | 86 (7.3) | 2 (0.2) | 9 (0.8) | 75 (6.3) |
| Age (y) | |||||||
| 5–11 | 94,518 | 61 (64.5) | 59 (62.4) | 2 (2.1) | 0 | 1 (1.1) | 1 (1.1) |
| Dose 1 | 58,636 | 47 (80.2) | 45 (76.7) | 2 (3.4) | 0 | 1 (1.7) | 1 (1.7) |
| Dose 2 | 35,882 | 14 (39.0) | 14 (39.0) | 0 | 0 | 0 | 0 |
| 12–17 | 4,900,762 | 14,725 (300.5) | 14,275 (291.3) | 450 (9.2) | 5 (0.1) | 100 (2.0) | 345 (7.0) |
| Dose 1 | 2,496,959 | 6,852 (274.4) | 6,614 (264.9) | 238 (9.5) | 0 | 81 (3.2) | 157 (6.3) |
| Dose 2 | 2,403,803 | 7,873 (327.5) | 7,661 (318.7) | 212 (8.8) | 5 (0.2) | 19 (0.8) | 188 (7.8) |
Data are presented as n (per 100,000): the reporting rate of adverse events per 100,000 doses administered.
CVMS, COVID-19 vaccination management system; COVID-19, coronavirus disease 2019.
a) Data were based on suspected adverse events following COVID-19 vaccination reported by medical institutions or doctors. The results do not indicate medically confirmed diagnoses or causality between the events and the vaccines.
b) Non-serious adverse events include common symptoms such as redness at the injection site, pain, swelling, myalgia, fever, headache, chills, and others.
c) Serious adverse events include the following: death, suspected anaphylaxis, and others.
d) Others include major adverse events including adverse events of special interest, intensive care unit admission, life-threatening events, permanent disability or sequelae, and others.
| Symptoms and signs (n=14,786)a) | Case (per 100,000) |
|---|---|
| Non-serious adverse events (n=14,334) | |
| Headache | 3,765 (75.4) |
| Chest pain | 3,417 (68.4) |
| Myalgia | 2,152 (43.1) |
| Dizziness | 2,065 (41.3) |
| Nausea | 1,843 (36.9) |
| Fever | 1,550 (31.0) |
| Allergic reactions | 918 (18.4) |
| Vomiting | 889 (17.8) |
| Abdominal pain | 872 (17.5) |
| Chills | 848 (17.0) |
| Pain, redness, or swelling at the injection site within 3 days after | 541 (10.8) |
| Diarrhea | 526 (10.5) |
| Lymphadenitis | 447 (8.9) |
| Abnormal uterine bleeding | 140 (2.8) |
| Cellulitis | 71 (1.4) |
| Arthritis | 59 (1.2) |
| Dyspneab) | 46 (0.9) |
| Severe local adverse events | 33 (0.7) |
| Itchingb) | 13 (0.3) |
| Abscess at the injection site | 2 (<0.1) |
| Systemic disseminated Bacillus Calmette-Guerin infection | 1 (<0.1) |
| Severe adverse events (n=452) including reports of death | |
| Acute cardiovascular injuryc) | 125 (2.5) |
| Anaphylaxisd) | 101 (2.0) |
| Convulsions or seizures | 49 (1.0) |
| Acute paralysis | 42 (0.8) |
| Vaccine-associated enhanced disease | 42 (0.8) |
| Acute respiratory distress syndrome | 18 (0.4) |
| Encephalopathy or encephalitis | 17 (0.3) |
| Thrombocytopenia | 7 (0.1) |
| Thrombocytopenic purpura | 6 (0.1) |
| Osteitis or osteomyelitis | 5 (0.1) |
| Anosmia or ageusia | 5 (0.1) |
| Erythema multiforme | 5 (0.1) |
| Coagulation disorder | 4 (0.1) |
| Acute kidney injury | 4 (0.1) |
| Single organ cutaneous vasculitis | 3 (0.1) |
| Multisystem inflammatory syndrome | 3 (0.1) |
| Acute liver injury | 3 (0.1) |
| Thrombosis | 3 (0.1) |
| Meningitis | 2 (<0.1) |
| Guillain-Barre syndrome | 2 (<0.1) |
| Myelitis | 1 (<0.1) |
| Capillary leak syndrome | 1 (<0.1) |
| Chilblains | 1 (<0.1) |
Data are presented as n (per 100,000): the reporting rate of adverse events per 100,000 doses administered.
CVMS, COVID-19 vaccination management system; COVID-19, coronavirus disease 2019.
a) Data were based on suspected adverse events following COVID-19 vaccination reported by medical institutions or doctors. The results do not indicate medically confirmed diagnoses or causality between the events and the vaccines.
b) These were reported from March 10, 2022.
c) Acute cardiovascular injury includes myocarditis, pericarditis, and others.
d) Anaphylaxis includes anaphylactoid reactions.
| Eventsa) | Dose 1 (n=11,414) | Dose 2 (n=3,688) | ||||
|---|---|---|---|---|---|---|
| 5–11 y (n=1,025) | 12–17 y (n=10,389) | p-valuec) | 5–11 y (n=541) | 12–17 y (n=3,147) | p-valuec) | |
| Local adverse events | 336 (32.8) | 5,009 (48.2) | <0.001 | 148 (27.4) | 1,672 (53.1) | <0.001 |
| Pain | 309 (30.1) | 4,612 (44.4) | <0.001 | 133 (24.6) | 1,546 (49.1) | <0.001 |
| Redness | 17 (1.7) | 234 (2.3) | 0.216 | 11 (2.0) | 108 (3.4) | 0.089 |
| Swelling | 58 (5.7) | 973 (9.4) | <0.001 | 29 (5.4) | 371 (11.8) | <0.001 |
| Itching | 30 (2.9) | 276 (2.7) | 0.609 | 11 (2.0) | 107 (3.4) | 0.095 |
| Urticaria | 5 (0.5) | 51 (0.5) | 1 | 1 (0.2) | 14 (0.4) | 0.712 |
| Others | 42 (4.1) | 592 (5.7) | 0.033 | 25 (4.6) | 195 (6.2) | 0.153 |
| Systemic adverse events | 275 (26.8) | 4,351 (41.9) | <0.001 | 121 (22.4) | 1,651 (52.5) | <0.001 |
| Fever | 94 (9.2) | 797 (7.7) | 0.088 | 48 (8.9) | 723 (23.0) | <0.001 |
| Chills | 55 (5.4) | 596 (5.7) | 0.625 | 21 (3.9) | 452 (14.4) | <0.001 |
| Headache | 97 (9.5) | 1,717 (16.5) | <0.001 | 40 (7.4) | 999 (31.7) | <0.001 |
| Joint pain | 15 (1.5) | 266 (2.6) | 0.031 | 3 (0.6) | 181 (5.8) | <0.001 |
| Myalgia | 132 (12.9) | 2,474 (23.8) | <0.001 | 48 (8.9) | 865 (27.5) | <0.001 |
| Fatigue or tiredness | 95 (9.3) | 2,091 (20.1) | <0.001 | 45 (8.3) | 892 (28.3) | <0.001 |
| Nausea | 37 (3.6) | 680 (6.5) | <0.001 | 14 (2.6) | 322 (10.2) | <0.001 |
| Vomiting | 13 (1.3) | 47 (0.5) | 0.001 | 5 (0.9) | 28 (0.9) | 0.809 |
| Diarrhea | 16 (1.6) | 224 (2.2) | 0.205 | 6 (1.1) | 81 (2.6) | 0.038 |
| Abdominal pain | 20 (2.0) | 379 (3.6) | 0.005 | 10 (1.8) | 171 (5.4) | <0.001 |
| Rash | 3 (0.3) | 35 (0.3) | 1 | 0 | 16 (0.5) | 0.151 |
| Armpit tenderness | 30 (2.9) | 412 (4.0) | 0.1 | 20 (3.7) | 327 (10.4) | <0.001 |
| Chest painb) | 8 (0.8) | - | - | 6 (1.1) | - | - |
| Heart palpitationsb) | 4 (0.4) | - | - | 1 (0.2) | - | - |
| Others | 42 (4.1) | 523 (5.0) | 0.187 | 19 (3.5) | 180 (5.7) | 0.036 |
| Unable to perform normal daily activities | 95 (9.3) | 1,052 (10.1) | 0.384 | 39 (7.2) | 613 (19.5) | <0.001 |
| Visits to medical facilities | 35 (3.4) | 116 (1.1) | <0.001 | 20 (3.7) | 44 (1.4) | <0.001 |
| Emergency department visit | 0 | 18 (0.2) | 0.399 | 0 | 7 (0.2) | 0.603 |
| Hospitalization | 0 | 2 (0) | 1 | 1 (0.2) | 0 | 0.147 |
| Clinic visit | 35 (3.4) | 100 (1.0) | <0.001 | 19 (3.5) | 39 (1.2) | <0.001 |
Data are presented as n (%): the percentage of respondents who reported adverse events and health conditions at least once during days 0 to 7 post-vaccination.
COVID-19, coronavirus disease 2019.
a) Events reported by respondents who completed at least 1 text message-based survey on days 0 to 7. Respondents were able to report multiple adverse events on each day.
b) These were additionally investigated only for children aged 5 to 11 years.
c) Chi-square or Fisher exact test as appropriate.
- 1. Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med 2022;386:35−46.ArticlePubMed
- 2. Frenck RW Jr, Klein NP, Kitchin N, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med 2021;385:239−50.ArticlePubMedPMC
- 3. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603−15.ArticlePubMedPMC
- 4. Ministry of Food and Drug Safety (MFDS). Press release: authorization for the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) [Internet]. Cheongju: MFDS; 2021 [cited 2022 Jul 7]. Available from: https://www.mfds.go.kr/brd/m_99/view.do?seq=45117&srchFr=&srchTo=&srchWord=&srchTp=&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&page=1. Korean.
- 5. Ministry of Food and Drug Safety (MFDS). Press release: age expansion to use of the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) [Internet]. Cheongju: MFDS; 2021 [cited 2022 Jul 7]. Available from: https://www.mfds.go.kr/brd/m_99/view.do?seq=45566&srchFr=&srchTo=&srchWord=접종+연령+확대&srchTp=0&itm_seq_1=0&itm_seq_2=0&multi_itm_seq=0&company_cd=&company_nm=&Data_stts_gubun=C9999&page=1. Korean.
- 6. Ministry of Food and Drug Safety (MFDS). Press release: authorization for the Pfizer-BioNTech COVID-19 vaccine (BNT162b2) for children aged 5 to 11 years old [Internet]. Cheongju: MFDS; 2022 [cited 2022 Jul 7]. Available from: https://nedrug.mfds.go.kr/bbs/119/201. Korean.
- 7. Korea Disease Control and Prevention Agency (KDCA). Press releases: adolescents aged 16-17 years vaccination (October 18, 2021, regular briefing) [Internet]. Cheongju: KDCA; 2021 [cited 2022 Oct 12). Available from: https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=717277&cg_code=C01&act=view&nPage=1. Korean.
- 8. Korea Disease Control and Prevention Agency (KDCA). Press releases: adolescents aged 12-17 years vaccination (August 30, 2021, regular briefing) [Internet]. Cheongju: KDCA; 2021 [cited 2022 Oct 12). Available from: https://www.kdca.go.kr/board/board.es?mid=a20501020000&bid=0015&list_no=716707&cg_code=C0&act=view&nPage=11. Korean.
- 9. Korea Disease Control and Prevention Agency (KDCA). Press releases: children aged 5-11 years vaccination (March 24, 2022, regular briefing) [Internet]. Cheongju: KDCA; 2021 [cited 2022 Oct 12). Available from: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=719074&cg_code=&act=view&nPage=1. Korean.
- 10. Korea Disease Control and Prevention Agency (KDCA). Press release: initiation of primary series vaccination for children (5–11 years old) and additional vaccination for adolescents (12–17 years old) [Internet]. Cheongju: KDCA; 2022 [cited 2022 Jul 7]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=718990&cg_code=&act=view&nPage=1. Korean.
- 11. Payne RP, Longet S, Austin JA, et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021;184:5699−714. e11.ArticlePubMedPMC
- 12. Buchan SA, Seo CY, Johnson C, et al. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Netw Open 2022;5:e2218505.ArticlePubMedPMC
- 13. Korea Disease Control and Prevention Agency (KDCA). Guideline for adverse events following COVID-19 immunization [Internet]. 2nd ed. Cheongju: KDCA; 2021 [cited 2022 Jul 7]. Available from: https://www.kdca.go.kr/upload_comm/syview/doc.html?fn=163463647072000.pdf&rs=/upload_comm/docu/0019. Korean.
- 14. Hause AM, Shay DK, Klein NP, et al. Safety of COVID-19 vaccination in United States children ages 5 to 11 years. Pediatrics 2022;150:e2022057313.ArticlePubMedPDF
- 15. Hause AM, Gee J, Baggs J, et al. COVID-19 vaccine safety in adolescents aged 12-17 years: United States, December 14, 2020-July 16, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1053−8.ArticlePubMedPMC
- 16. Hwang I, Park K, Kim TE, et al. COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021. Osong Public Health Res Perspect 2021;12:396−402.ArticlePubMedPMCPDF
- 17. Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US-December 14, 2020-January 18, 2021. JAMA 2021;325:1101−2.ArticlePubMedPMC
- 18. Bian S, Li L, Wang Z, et al. Allergic reactions after the administration of COVID-19 vaccines. Front Public Health 2022;10:878081. ArticlePubMedPMC
- 19. Wong HL, Hu M, Zhou CK, et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet 2022;399:2191−9.ArticlePubMedPMC
- 20. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. JAMA 2022;327:331−40.ArticlePubMedPMC
- 21. Lv M, Luo X, Shen Q, et al. Safety, Immunogenicity, and efficacy of COVID-19 vaccines in children and adolescents: a systematic review. Vaccines (Basel) 2021;9:1102. ArticlePubMedPMC
- 22. Ling RR, Ramanathan K, Tan FL, et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med 2022;10:679−88.ArticlePubMedPMC
- 23. Centers for Disease Control and Prevention (CDC). COVID-19: myocarditis and pericarditis after mRNA COVID-19 vaccination [Internet]. Atlanta, GA: CDC; 2021 [cited 2022 Jul 25]. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html.
- 24. Hause AM, Baggs J, Marquez P, et al. COVID-19 vaccine safety in children aged 5-11 years: United States, November 3-December 19, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1755−60.ArticlePubMedPMC
- 25. Su JR. Adverse events among children ages 5-11 years after COVID-19 vaccination: updates from v-safe and the Vaccine Adverse Event Reporting System (VAERS) [Internet]. Atlanta, GA: CDC; 2021 [cited 2022 Jul 7]. Available from: https://stacks.cdc.gov/view/cdc/112668.
- 26. Food and Drug Administration (FDA). Comirnaty and Pfizer-BioNTech COVID-19 vaccine [Internet]. Silver Spring, MD: FDA; 2021 [cited 2022 Jul 7]. Available from: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine.
- 27. Woodworth KR, Moulia D, Collins JP, et al. The advisory committee on immunization practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine in children aged 5-11 years: United States, November 2021. MMWR Morb Mortal Wkly Rep 2021;70:1579−83.ArticlePubMedPMC
- 28. Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: update from the advisory committee on immunization practices: United States, June 2021. MMWR Morb Mortal Wkly Rep 2021;70:977−82.ArticlePubMedPMC
- 29. Wise J. Covid-19: should we be worried about reports of myocarditis and pericarditis after mRNA vaccines? BMJ 2021;373:n1635. ArticlePubMed
- 30. Saxena S, Skirrow H, Wighton K. Should the UK vaccinate children and adolescents against COVID-19? BMJ 2021;374:n1866. ArticlePubMed
- 31. Ryan M, Montgomery J. Myopericarditis after COVID-19 vaccination: unexpected but not unprecedented. Lancet Respir Med 2022;10:625−5.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Safety monitoring of COVID-19 vaccines: February 26, 2021, To June 4, 2022, Republic of Korea
Yeon-Kyeng Lee, Yunhyung Kwon, Yesul Heo, Eun Kyoung Kim, Seung Yun Kim, Hoon Cho, Seontae Kim, Mijeong Ko, Dosang Lim, Soon-Young Seo, Enhi Cho
Clinical and Experimental Pediatrics.2023; 66(10): 415. CrossRef - Effectiveness of the BNT162b2 vaccine in preventing morbidity and mortality associated with COVID-19 in children aged 5 to 11 years: A systematic review and meta-analysis
Sumayyah Ebrahim, Ntombifuthi Blose, Natasha Gloeck, Ameer Hohlfeld, Yusentha Balakrishna, Rudzani Muloiwa, Andy Gray, Andy Parrish, Karen Cohen, Ruth Lancaster, Tamara Kredo, Julia Robinson
PLOS Global Public Health.2023; 3(12): e0002676. CrossRef


 Cite
Cite