Search
- Page Path
- HOME > Search
Brief Report
- JYNNEOS vaccine safety monitoring in the Republic of Korea, 2022: a cross-sectional study
- Jaeeun Lee, Seunghyun Lewis Kwon, Jinhee Park, Hyuna Bae, Hyerim Lee, Geun-Yong Kwon
- Osong Public Health Res Perspect. 2023;14(5):433-438. Published online October 18, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0182
- 942 View
- 38 Download
- 1 Web of Science
- 1 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 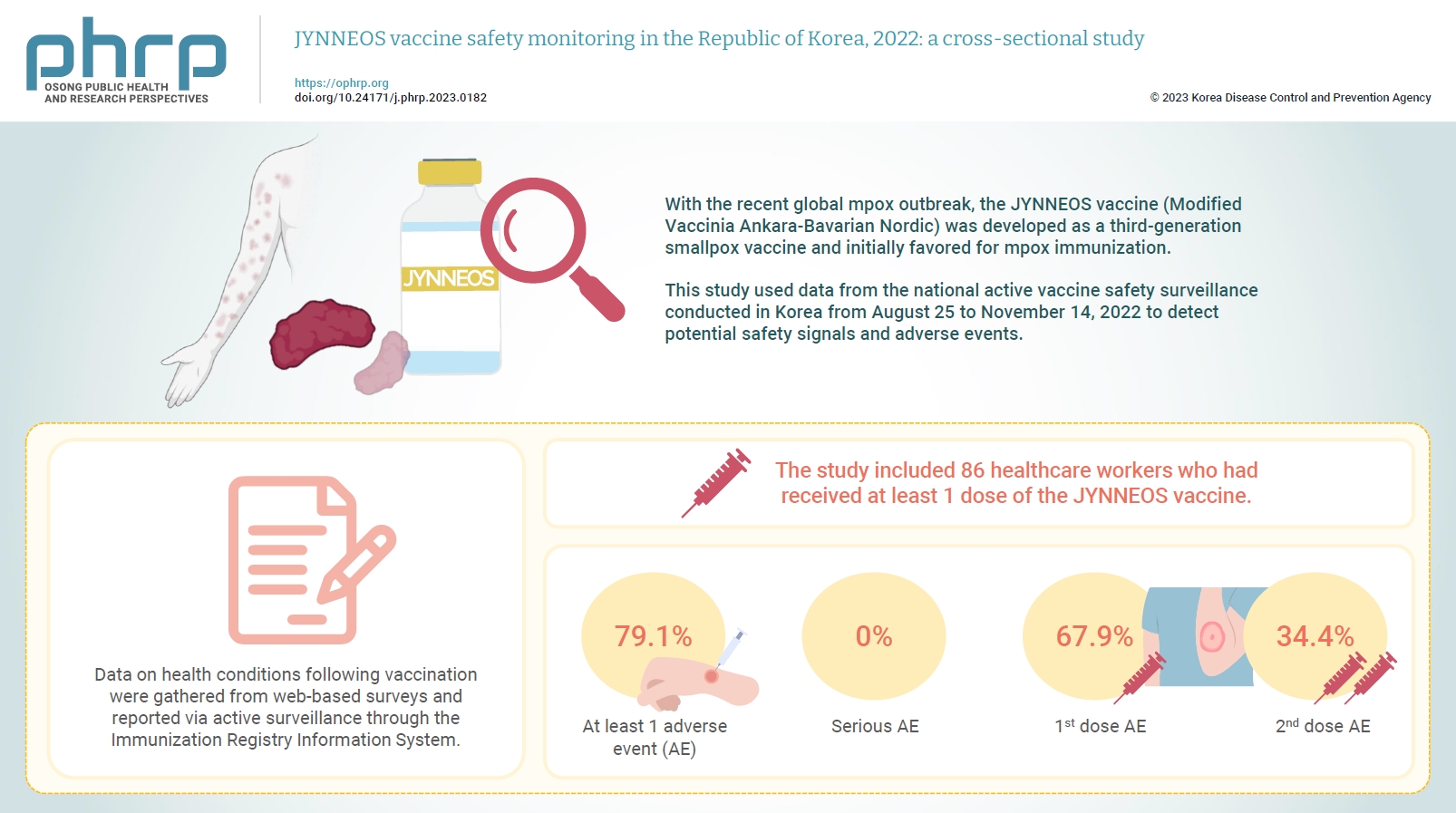
- Objectives
With the recent global mpox outbreak, the JYNNEOS vaccine (Modified Vaccinia Ankara-Bavarian Nordic) was developed as a third-generation smallpox vaccine and initially favored for mpox immunization. Vaccine-associated side effects contribute to vaccine hesitancy. Consequently, tracking adverse events post-immunization is crucial for safety management. This study used data from the national active vaccine safety surveillance conducted in Korea from August 25 to November 24, 2022 to detect potential safety signals and adverse events. Methods: Data on health conditions following vaccination were gathered from web-based surveys and reported via active surveillance through the Immunization Registry Information System. This follow-up system functioned via a text message link, surveying adverse events and health conditions beginning on the second day post-vaccination. Information about specific adverse events, including both local and systemic reactions, was collected. Results: The study included 86 healthcare workers who had received at least 1 dose of the JYNNEOS vaccine. Among the respondents, 79.1% reported experiencing at least 1 adverse event, with the majority being local reactions at the injection site. The incidence of adverse events was higher following the first dose (67.9%) than after the second dose (34.4%). The most frequently reported adverse event for both doses was mild pain at the injection site. Conclusion: The study provides crucial information on the safety of the JYNNEOS vaccine, demonstrating that most adverse events were manageable and predominantly localized to the injection site. Nonetheless, additional research is needed on the safety of various vaccine administration techniques and the vaccine’s effects on broader demographics. -
Citations
Citations to this article as recorded by- Adverse Reactions After Intradermal Vaccination With JYNNEOS for Mpox in Korea
So Yun Lim, Yu Mi Jung, Yeonjae Kim, Gayeon Kim, Jaehyun Jeon, BumSik Chin, Min-Kyung Kim
Journal of Korean Medical Science.2024;[Epub] CrossRef
- Adverse Reactions After Intradermal Vaccination With JYNNEOS for Mpox in Korea
Original Article
- Prevalence and patterns of adverse events following childhood immunization and the responses of mothers in Ile-Ife, South West Nigeria: a facility-based cross-sectional survey
- Olorunfemi Akinbode Ogundele, Funmito Omolola Fehintola, Mubarak Salami, Rahmat Usidebhofoh, Mary Aderemi Abaekere
- Osong Public Health Res Perspect. 2023;14(4):291-299. Published online July 27, 2023
- DOI: https://doi.org/10.24171/j.phrp.2023.0071
- 2,303 View
- 135 Download
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 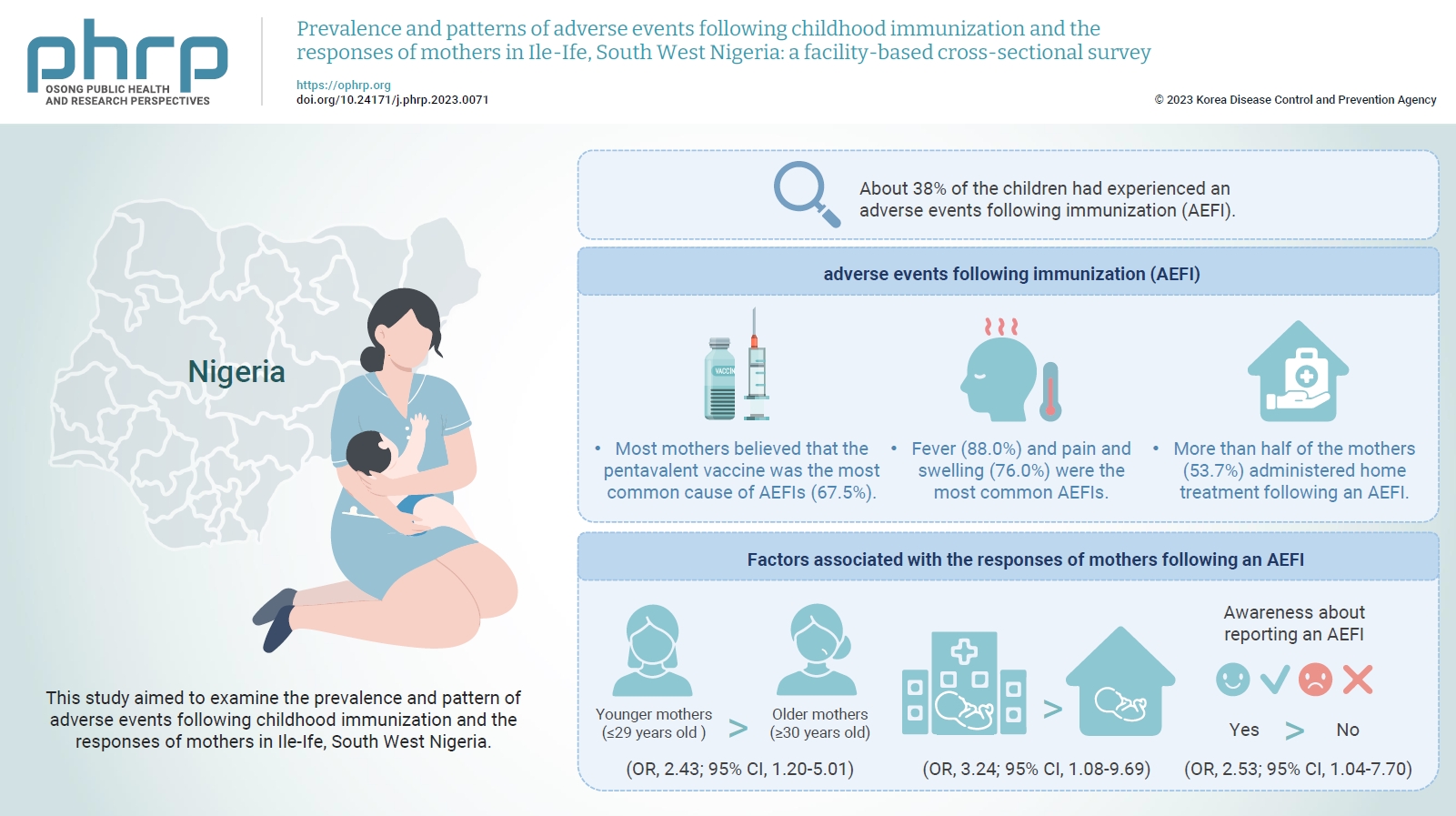
- Objectives
This study aimed to examine the prevalence and pattern of adverse events following childhood immunization and the responses of mothers in Ile-Ife, South West Nigeria.
Methods
This descriptive cross-sectional study was conducted among 422 mothers of children aged 0 to 24 months attending any of the 3 leading immunization clinics in Ile-Ife, Nigeria. The respondents were selected using the multi-stage sampling technique. Data were collected using a pretested structured interviewer-administered questionnaire and analyzed using IBM SPSS ver. 26.0. The chi-square test was used to test associations, while binary logistic regression was used to determine the predictors of mothers’ responses to adverse events following immunization (AEFIs). A p-value of <0.05 was considered statistically significant.
Results
The mean age of the respondents was 29.99±5.74 years. About 38% of the children had experienced an AEFI. Most mothers believed that the pentavalent vaccine was the most common cause of AEFIs (67.5%). Fever (88.0%) and pain and swelling (76.0%) were the most common AEFIs. More than half of the mothers (53.7%) administered home treatment following an AEFI. Younger mothers (odds ratio [OR], 2.43; 95% confidence interval [CI], 1.20–5.01), mothers who delivered their children at a healthcare facility (OR, 3.24; 95% CI, 1.08–9.69), and mothers who were knowledgeable about reporting AEFIs (OR, 2.53; 95% CI, 1.04–7.70) were most likely to respond appropriately to AEFIs.
Conclusion
The proportion of mothers who responded poorly to AEFIs experienced by their children was significant. Therefore, strategies should be implemented to improve mothers’ knowledge about AEFIs to improve their responses.



 First
First Prev
Prev


