Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 3(3); 2012 > Article
-
Articles
Surveillance and Vector Control of Lymphatic Filariasis in the Republic of Korea - Shin Hyeong Choa, Da Won Maa, Bo Ra Kooa, Hee Eun Shina, Wook Kyo Leeb, Byong Suk Jeonga, Chaeshin Chuc, Won Ja Leea, Hyeng Il Cheuna
-
Osong Public Health and Research Perspectives 2012;3(3):145-150.
DOI: https://doi.org/10.1016/j.phrp.2012.07.008
Published online: June 30, 2012
aDivision of Malaria and Parasitic Diseases, Korea National Institute of Health, Osong, Korea.
bDivision of Medical Entomology, Korea National Institute of Health, Osong, Korea.
cDivision of Epidemic Intelligence Service, Korea Centers for Disease Control and Prevention, Osong, Korea.
- Corresponding author. E-mail: ilcheun7@korea.kr
• Received: July 3, 2012 • Revised: July 19, 2012 • Accepted: July 19, 2012
Copyright ©2012, Korea Centers for Disease Control and Prevention
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License () which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives
- Until the early 2000s, lymphatic filariasis would commonly break out in the coastal areas in Korea. Through steady efforts combining investigation and treatment, filariasis was officially declared eradicated in 2008. This study surveyed the density of vector species of filariasis in past endemic areas, and inspected filariasis DNA from collected mosquitoes for protection against the reemergence of filariasis.
-
Methods
- Between May and October 2009, mosquitoes were caught using the black night trap in past endemic coastal areas: Gyeongsangnam-do, Jeollanamdo, and Jeju-do. The collected mosquitoes were identified, and the extracted DNA from the collected vector mosquitoes was tested by polymerase chain reaction for Brugia malayi filariasis.
-
Results
- Ochletotatus togoi, Anophel es (Hyrcanus) group and Culex pipiens were most frequently caught in Jeollanam-do (Geomun Island, Bogil Island, Heuksan Island), Jeju-do (Namone-ri, Wimi-ri). and Gyeongsangnam-do (Maemul Island). DNA of B malayi was not found in Och Togoi and An (Hyrcanus) group as main vectors of filariasis.
-
Conclusion
- Lymphatic filariasis was not found in the vector mosquitoes collected in past endemic areas. However, considering that the proportion of vector species is quite high, there is a potential risk that filariasis could be reemerging through overseas travel or trade. Thus, there is a need to continuously monitor vector mosquitoes of lymphatic filariasis.
- Lymphatic filariasis is a vector-borne nematode infection caused by Wucherea bancrofti, Brugia malayi and Brugia timori, and more than 100 million people are affected worldwide [1,2]. Of this total, 90% are caused by W bancrofti and 10% by B malayi [3]. The first case of elephantiasis in Korea was reported in 1927 [4], and it was called by various names: Soojongdari (leg dropy), Pinaerim (blood down flow), Pijoeng (blood diseases), and Gakmomsal (malaise with arthralgia) [5]. In the 1940s, the infection rate reached as high as 12–26% in the southern parts of the country and Jeju-do [6-8]. The rate remained at a 12% average until the early 1970s (range, between 5.5% and 18.0%) in the more inland areas of Youngju-si (si = district), Gyeongsangbuk-do (do = province). Following the government’s active efforts for treatment, the disease was eliminated in the late 1980s [9-11]. By the mid-1980s, the average rate fell below 1% even in Jeju-d,o where the infection was most prevalent [12]. Furthermore, no positive cases of filariasis were reported in past endemic areas of Jeollanam-do, Gyeongsan-do, and Jeju-do between 2002 and 2006 [13]. Finally, the World Health Organization verified that filariasis was eliminated in Korea, declaring the country free from lymphatic filariasis [13-15]. However, monitoring of vector mosquitoes should continue to prevent possible reemergence due to increasing overseas travel and climate changes. In this study, we investigated the density and distribution of
- vector mosquitoes of filariasis, and its DNA from vector mosquitoes was tested.
1. Introduction
- 2.1. Survey areas
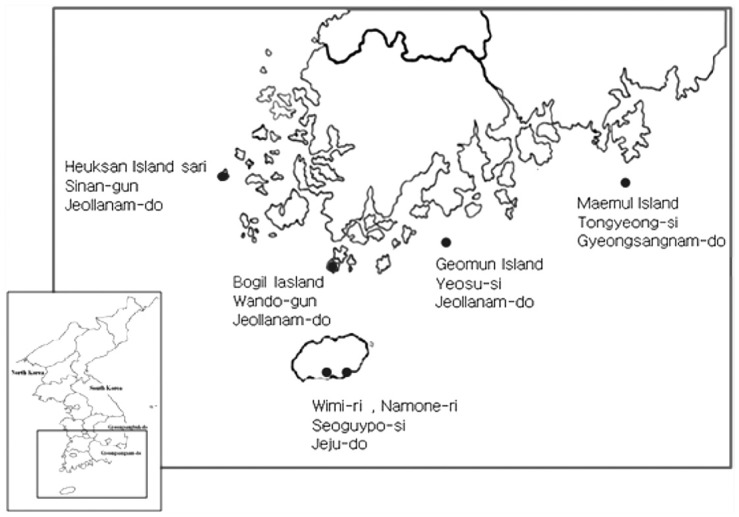
- Jeollanam-do [Sa-ri (ri = village), Heuksan Island in Shinan-gun, Geomun-ri, Geomun Island in Yeosu-si (si = district), Baekdo-ri, Bogil Island in Wando-gun], Gyeongsangnam-do (Maejuk-ri, Maemul Island in Tongyoung-si), and Jeju-do (Island) (Wimi-ri, Seoguipo-si) were surveyed (Figure 1).
- 2.2. Adult mosquito surveillance
- Adult mosquitoes were collected twice weekly using the commercial Black Hole® mosquito black light trap (Model: Black Hole, Bio-trap Inc., Seoul, Korea). The traps were placed under the eaves of houses from 19:00 to 06:00 hours the following morning. All mosquitoes were identified to the genus or species level under a dissecting microscope using standard morphological keys [16,17]. An sinensis was assigned to the Anopheles (Hyrcanus) group, because of the difficulty in identifying the species by microscope.
- 2.3. DNA extraction and PCR condition
- The selected Och togoi and An (Hyrcanus) Group were pooled to 1–3 mosquitoes, and total DNA was extracted with the QIAamp DNA kit according to the manufacturer’s protocol (Qiagen, Valencia, CA, USA). Hha1 F 5'-
- Seasonal variations of mosquitoes collected in mosquito trap in Sa-ri, Heuksan lsland (latitude 34°39'N, longitude 125°25'E), Shinan-gun, Jeollanam-do in 2009
- GCGCATAAATTCATCAGC-3', R 5'-GCGCAAAAC TTAATTACAAAAGC-3' of two set primers were used [18]. Polymerase chain reaction (PCR) was performed in a 25-μL reactive solution containing 5 μL of DNA template, 20 pmol of each primer, 1.25 U of ExTaq DNA polymerase (Takara Co., Japan), 2 mM MgCl2, and 250 μM of each dNTP. The following PCR cycle was performed in an iCycler thermal cycler (Bio-Rad Ltd., Hercules, CA, USA): 1 × 94℃ for 5minutes, 40 × (94℃ for 1 minute, 56℃ for 1 minute, 72℃ for 1 minute), 1 × 72℃ for 10 minutes.
2. Materials and Methods
Table 1.
- 3.1. Sa-ri, Heuksan lsland, Shinan-gun
- A total of 149 mosquitoes were collected in Heuksan Island, consisting of four genera and six species. Among them, Och togoi was the most common (62.4%), followed by Culex pipiens (16.8%), Armigeres subalbatus (13.4%), Culex tritaeniorhynchus (6%), Culex bitaenirohynchus (0.7%), and An (Hyrcanus) Group (0.7%) (Table 1). However, filariasis DNA was not detected in the vector species of Och togoi and An (Hyicanus) group.
- 3.2. Baekdo-ri, Bogil lsland, Wando-gun
- In Bogil Island, 3097 mosquitoes were collected in seven genera and 12 species. Och togoi was the most
- Seasonal variations of mosquitoes collected in mosquito trap in Baekdo-ri, Bogil lsland (latitude 34°08'N, longitude 126°32'E), Wando-gun, Jeollanam-do, in 2009
- frequent (84.1%), followed by An (Hyrcanus) Group (5.3%), Cx tritaeniorhynchus (4.1%), Ar subalbatus (2.5%), Cx pipiens (1.3%), Och dorsalis (1.1%), An sinesis (1.0%), Coh ochracea (0.3%), Och nipponicus (0.1%), Ae vexans (0.03%), and Lutrie vorax (0.03%). The number of collected Och togoi rose sharply between May and June, and remained high until September. Neither Och Togoi nor An (Hyicanus) group had filariasis DNA (Table 2).
- 3.3. Geomun-ri, Geomun lsland, Yeosu-si
- A total of 376 mosquitoes were collected in Geomunri, Geomun Island, comprising five genera and eight species. Among them, the number of Cx pipiens was highest (45.2%), followed by Och togoi (42%), Ar subalbatus (6.4%), Cx triraenior (4.8%), Ae albopirtus (0.8%), Cx inatomi (0.3%), Och koreicus (0.3%), and Lu vorax (0.3%) (Table 3). Filariasis DNA was not found in Och togoi and An (Hyicanus) group.
- 3.4. Maejuk-ri, Maemul lsland, Tongyoung-si
- A total of 1213 mosquitoes were collected in Maejuk-ri, Maemul Island, comprising of four genera and seven species. Och togoi was most frequently caught (84.9%), followed by Ar subalbatus (12%), Cx pipiens (2.1%), Cx triraenior (0.5%), Ae albopirtus (0.3%), Och koreicus (0.2%), and Cx bitaenior (0.1%). The number of Och togoi picked up sharply between May and June, and dropped significantly between
- Seasonal variations of mosquitoes collected in mosquito trap in Geomun-ri (latitude 34°17'N, longitude 127°23'E), Geomun lsland, Yeosu-si, Jeollanam-do in 2009
- September and October (Table 4). Filariasis DNA was not found in Och togoi and An (Hyicanus) group.
- 3.5. Namone-ri, Seoguipo-si
- A total of 247 mosquitoes were collected in Namoneri, Seoguipo-si, Jeju-do, comprising two genera and four species. Cx pipiens was most frequently caught (81.8%), followed by Och togoi (13%), Cx triraenior (4.9%), and Cx mineficus (0.4%). No filariasis DNA was detected in Och togoi and An (Hyicanus) group (Table 5).
- 3.6. Wimi-ri, Seoguipo-si
- A total of 298 mosquitoes were collected in Wimi-ri, Seoguipo-si, Jeju-do, comrpsing four genera, five species. Cx pipiens was most frequently caught (77.9%), followed by Och togoi (15.8%), Cx triraenior (5.4%), Ar subalbatus (0.7%), and Ae albopirtus (0.3%). No filariasis DNA was detected in Och togoi and An (Hyicanus) group (Table 6).
3. Results
Table 2.
Table 3.
- In the survey, the ratio of Och togoi was highest in Maemul Island, Bogil Island, and Heuksan Island, while Cx pipiens was most frequently caught in Geomun Island, Namone-ri, and Wimi-ri in Jeju-do. This trend remained consistent in the survey between 2002 and 2005 by Cheun et al [19], and Cx pipiens also found out
- Seasonal variations of mosquitoes collected in mosquito trap in Maejuk-ri (latitude 34°38'N, longitude 128°34'E), Maemul lsland, Tongyoung-si, Gyeongsangnam-do in 2009
- to be the dominant species in Geomun Island and Jejudo. According to Ree [20], there are nine genera of mosquitoes found in Korea: Anopheles, Culex, Ochletotatus (formerly known as Aedes), Armigeres, Mansonia, Heizmannia, Tripteroides, Culisera, and Tozorhynchites. Among them, Och togoi was recognized as the main vector mosquito of B malayi in Jeju-do, Korea [8,21], whereas An sinensis sensu stricto, associated with rice paddies, is found in inland areas [9,14].
- According to Lee [22], two larvae from mosquitoes were found to be infected with B malayi in 464 Aedes togoi collected in Jeju-do; after this, several researchers found that the natural infection rate of Aedes togoi was between 1.4% and 10.7%, and Och togoi was a vector of Malayan filariasis in Jeju-do [23-25]. However, Ae koreicus and Cu pipens were reported to have poor susceptibility as a vector of filariasis [26]. Furthermore, Kim et al [10] also collected 4351 An sinesis in Youngju-si, Chungcheongbuk-do, inland areas, and found that 14 mosquitoes (0.3%) were infected with B malayi. Therefore, these studies showed that both Och togoi and An sinensis had susceptibility for lymphatic filariasis [10]. In this study, An (Hyrcanus) group and Och togoi collected in these past endemic areas, however, were not infected with B malayi, and this fact is proof that these areas are free of lymphatic filariasis.
- Next, mosquito larvae are susceptible to salinity and temperature, and their numbers increase after the
- Seasonal variations of mosquitoes collected in mosquito trap in Namone-ri (latitude 33°16'N longitude 126°39'E), Seoguipo-si, Jeju-do in 2009
- Seasonal variations of mosquitoes collected in mosquito trap in Wimi-ri (latitude 33°16'N, longitude 126°39'E), Seoguipo-si, Jeju-do in 2009
- summer monsoon season [19]. As the survey results show, the number began to climb up in June in all survey areas, stayed high July through September, and started to fall in October. According to Nakamura (1988), the number of Och togoi larvae begins to increase slowly when winter is over, and peaks in early May. The number falls in summer as the air and water temperature rises with strong sunlight. However, when rainfall increases in June through September, the number increases again due to lower salinity in rock pools [27]. This means that seasonal condition and regional characteristics have a direct influence on the number of mosquito larvae. Compared to a previous study [21], the regional decrease or increase in the number of collected mosquitoes was affected by these conditions. However, this study confirmed that Och togoi and An (Hyrcanus) group are the dominant species in survey areas.
- This is the first study conducted in Korea that investigated filariasis DNA from vector mosquitoes in six remote island areas in Korea, and the negative results indicate that filariasis has been eliminated in the country. In particular, the costal island areas in Jeollanam-do showed a high infection rate until the early 2000s [28], but government, academic, and local clinics have been exerting efforts continuously to bring about its elimination. As the country’s economic growth accelerated from the late 1980s, peoples’ quality of living improved. They had better access to medical treatment, and various mosquito-repelling chemicals and equipment were developed such as mosquito nets. All these factors substantially reduced human contact with mosquitoes [29].
- Finally, the survey examined the number of mosquitoes, including vector species for filariasis, in island areas of Korea, and confirmed the negative result in DNA detection. However, filariasis has a latent period of 4–10 years, and there is a possibility of its emergence or reemergence through travel or trade as more people visit areas in the West Pacific, Southeast Asia, and Africa, where filariasis is still active. For this reason, it is important to continue quarantine inspection and monitoring of vector mosquitoes.
4. Discussion
Table 4.
Table 5.
| Month | Cx mineficus | Cx pipiens | Cx tritaenior | Och togoi | Mean no./trap |
|---|---|---|---|---|---|
|
|
|||||
| Jun | 9 | 9 | 18 | ||
| Jul | 56 | 2 | 7 | 65 | |
| Aug | 52 | 4 | 56 | ||
| Sep | 53 | 10 | 9 | 72 | |
| Oct | 1 | 32 | 3 | 36 | |
| Total (%) | 1 (0.4) | 202 (81.8) | 12 (4.9) | 32 (13) | 247 |
Table 6.
-
Acknowledgements
- We thank our colleagues at the Province and City Bureau of Health Center and the Research Institute of Health and Environment for their devoted support and efforts. This work was supported by a grant from Korea National Institute of Health (NIH-091-4800-4845-300), National Research and Development Program, Ministry of Health and Welfare, the Republic of Korea.
- 1. Michael E Bundy DA Grenfell BT. . Re-assessing the global prevalence and distribution of lymphatic filariasis. Parasitology 1996;112(Pt 4). 409−28. PMID: 8935952.ArticlePubMed
- 2. Ottesen EA. . Lymphatic filariasis: treatment, control and elimination. Adv Parasitol 2006;61:395−441. PMID: 16735170.ArticlePubMed
- 3. World Health Organization.Lymphatic filariasis. Wkly Epidemiol Rec 2003;78:169−80. PMID: 12808787.PubMed
- 4. Yun IS. . Elephantiasis due to filaria in Korea. Chosen Iggakai Zasshi 1927;76:326−34.
- 5. Seo BS. . Malayan filariasis in Korea. Korean J Parasitol 1978;16(Suppl.). 5−108.Article
- 6. Seo BS. . Studies on filariasis in Korea: status survey and chemotherapy in Cheju Do. Seoul J Med 1976;17:83−95.
- 7. Senoo T Lincicome DR. . Malayan filariasis. Incidence and distribution in Southern Korea. U.S. Armed Forces Med J 1951;2(10). 1483−9.
- 8. Lee KT Kim SH Kong TH et al.. Malayan filariasis. 2nd report: epidemiological investigations on filariasis due to Brugia malayi in the residents of southern Cheju-Do island. J Korean Med Assoc 1964;7:657−64.
- 9. Kim DC Lee OY Lee KW. . Epidemiology of malayan filariasis of inland Korea: 1. endemicity of filariasis malayi in Yongju area. Yonsei Rep Trop Med 1977;8:9−22.
- 10. Kim DC Lee OY Lee KW. . Epidemiology of malayan filariasis of inland Korea: II. Vector finding and transmission of Brugia malayi in Yongju area. Yonsei Rep Trop Med 1977;8:23−32.
- 11. Lee JS Kim TS Lee WJ et al.. Epidemiology of malayan filariasis of inland Korea (III). Report of NIH Korea 1992;29:114−22.
- 12. Lee OY Lee JS Son SC et al.. Epidemiological studies on filariasis malayi on Cheju Do and the southern islands. Report NIH Korea 1986;23:407−22.
- 13. Cheun HI Lee JS Cho SH et al.. Elimination of lymphatic filariasis in the Republic of Korea: an epidemiological survey of formerly endemic areas, 2002–2006. Trop Med Int Health 2009;14(4). 445−9. PMID: 19228352.ArticlePubMed
- 14. Kim DC. . Epidemiological studies of filariasis in inland Korea: 4. Vector determination of filariasis malayi in Yongju Area. Abstracts of the 16th Annual Meeting of the Korean Society for Parasitology. 1974.
- 15. Cheun HI Cho SH Lee JS et al.. Successful control of lymphatic filariasis in the Republic of Korea. Korean J Parasitol 2009;47(4). 323−35. PMID: 19967079.ArticlePubMedPMC
- 16. Tanaka K Mizusawa K Saugstad ES. . Mosquitoes of Japan and Korea. Contrib Am Entomol Inst 1979;16:148−52.
- 17. Lee KW. . A revision of the illustrated taxonomic keys to genera and species of female mosquitoes of Korea. In: Department of the Army, 5th Medical Detachment, 168th Medical Battalion, 18th Medical Command; 1998.
- 18. Mishra K Kumar Raj D Dash AP et al.. Combined detection of Brugia malayi and Wuchereria bancrofti using single PCR. Acta Trop 2005;93(3). 233−7. PMID: 15715996.ArticlePubMed
- 19. Cheun HI Cho SH Lee HI et al.. Seasonal prevalence of mosquitoes, including vectors of brugian filariasis, in southern islands of the Republic of Korea. Korean J Parasitol 2011;49(1). 59−64. PMID: 21461270.ArticlePubMedPMC
- 20. Ree HI. . Medical entomology.. Komoon Sa; Seoul, Korea: 1978. pp 1−294.
- 21. Kim JS Lee WY Chun SL. . Ecology of filariasis on Cheju Island. Korean J Parasitol 1973;11(1). 33−53.Article
- 22. Lee WY. . A study on Aedes togoi as vector of filariasis in Cheju Island. Korean J Parasitol 1969;7(2). 153−9.Article
- 23. Chun SR. . A preliminary survey of mosquitoes of Cheju do related to filariasis, on the species, biology and infection status. Korean J Public Health 1968;5:113−21.
- 24. Wada Y Katamine D Oh MY. . Studies on malayan filariasis in Cheju Island, Korea: 2. Vector mosquitoes of malayan filariasis. Jpn J Trop Med Hyg 1973;1(3-4). 197−210.Article
- 25. Seo BS. . The periodicity of microfilariae of Brugia malayi in Cheju Island, Korea. Korean J Parasitol 1974;12(2). 95−100.Article
- 26. Kim DC Lee OY Kim TW et al.. Epidemiological studies of human filariasis of inland Korea: endemicity and transmission of human filariasis in Yongju area. Report NIH Korea 1971;8:147−65.
- 27. Nakamura S Miyagi I Toma T. . Seasonal appearance of immature populations of Aedes (Finlaya) togoi (Theobald) in Okinawa. Jpn J Sanit Zool 1988;39(2). 91−6.Article
- 28. Chai JY Lee SH Choi SY et al.. A survey of Brugia malayi infection on the Heuksan islands, Korea. Korean J Parasitol 2003;41(1). 69−73. PMID: 12666733.ArticlePubMedPMC
- 29. Korea National Institute of Health, Centers for Disease Control and Prevention.2007 Elimination of Lymphatic filariasis in Korea (National Documentation for Certification) 2007;1−149.
Figure & Data
References
Citations
Citations to this article as recorded by 

- The new invasive mosquito species Aedes koreicus as vector-borne diseases in the European area, a focus on Italian region: What we know from the scientific literature
Sonia Ganassi, Antonio De Cristofaro, Dalila Di Criscio, Sonia Petrarca, Chiara Leopardi, Antonio Guarnieri, Laura Pietrangelo, Noemi Venditti, Roberto Di Marco, Giulio Petronio Petronio
Frontiers in Microbiology.2022;[Epub] CrossRef - Monitoring migrant groups as a post-validation surveillance approach to contain the potential reemergence of lymphatic filariasis in Togo
Monique Ameyo Dorkenoo, Martin Kouame Tchankoni, Degninou Yehadji, Kossi Yakpa, Mawèké Tchalim, Efoe Sossou, Rachel Bronzan, Didier Koumavi Ekouevi
Parasites & Vectors.2021;[Epub] CrossRef - Geographical Genetic Variation and Sources of Korean Aedes albopictus (Diptera: Culicidae) Populations
EunJung Lee, Seong-Chan Yang, Tae-Kyu Kim, Byung-Eon Noh, Hak Seon Lee, Hyunwoo Kim, Jong Yul Roh, Wook-Gyo Lee, Michel Slotman
Journal of Medical Entomology.2020; 57(4): 1057. CrossRef - A systematic review of alternative surveillance approaches for lymphatic filariasis in low prevalence settings: Implications for post-validation settings
Nicholas Riches, Xavier Badia-Rius, Themba Mzilahowa, Louise A. Kelly-Hope, Patrick J. Lammie
PLOS Neglected Tropical Diseases.2020; 14(5): e0008289. CrossRef - An Insight into the Discovery of Potent Antifilarial Leads Against Lymphatic Filariasis
Pone Kamdem Boniface, Ferreira Igne Elizabeth
Current Drug Targets.2020; 21(7): 657. CrossRef - Prevention and Control Strategies for Parasitic Infections in the Korea Centers for Disease Control and Prevention
Young Yil Bahk, Eun-Hee Shin, Shin-Hyeong Cho, Jung-Won Ju, Jong-Yil Chai, Tong-Soo Kim
The Korean Journal of Parasitology.2018; 56(5): 401. CrossRef - Phylogeography of the Coastal Mosquito Aedes togoi across Climatic Zones: Testing an Anthropogenic Dispersal Hypothesis
Teiji Sota, Peter Belton, Michelle Tseng, Hoi Sen Yong, Motoyoshi Mogi, Igor Mokrousov
PLOS ONE.2015; 10(6): e0131230. CrossRef


 PubReader
PubReader Cite
Cite
