Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 3(2); 2012 > Article
-
Articles
Non-chromatographic Method for the Hepatitis B Virus X Protein Using Elastin-Like Polypeptide Fusion Protein - Soon-Hwan Kwona,b, Hyeseong Chob
-
Osong Public Health and Research Perspectives 2012;3(2):79-84.
DOI: https://doi.org/10.1016/j.phrp.2012.04.003
Published online: June 30, 2012
aDivision of High-Risk Pathogen Research, Korea National Institute of Health, Osong, Korea
bDepartment of Biochemistry, School of Medicine, Ajou University, Suwon, Korea.
- Corresponding author. E-mail: ichkann1472@kg21.net
• Received: December 16, 2011 • Revised: January 15, 2012 • Accepted: January 20, 2012
Copyright ©2012, Korea Centers for Disease Control and Prevention
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License () which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Objectives
- Hepatitis B virus (HBV) is a member of the hepadnavirus family. The HBV genome contains four genes designated as S, C, P, and X. The HBV X (HBx) gene encodes for a 16.5-kDa regulatory protein that enhances HBV replication and exerts multifunctional activities. The aim of this study is to describe the rapid and easy purification of HBx using ELP (elastin-like polypeptide) fusion protein.
-
Methods
- The ELP–HBx fusion protein was overexpressed in Escherichia coli. Environmental sensitivity was demonstrated via turbidity and dynamic light scattering as a function of temperature. HBx was purified as an ELP fusion protein. ELPs are biopolymers of the pentapeptide repeat Val-Pro-Gly-Xaa-Gly that undergo an inverse temperature phase transition. ELP follows in temperature and salt consistency, precipitation, and solution repetition (inverse transition cycling) with polypeptide, where it purifies the protein in a simple manner.
-
Results
- Fusion proteins underwent supramolecular aggregation at 40 ℃ in 1 M NaCl and slowly resolubilized at subphysiologic temperatures. ELP domain proteolysis liberated a peptide of comparable size and immunoreactivity to the commercial HBx.
-
Conclusion
- This study suggests that HBx can be purified rapidly and easily using inverse transition cycling, and that this method can be applied in determination of HBx 3D structures and HBx stability study.
- In alcohol or virus infection, persistent hepatic inflammation precedes chronic liver injury. A series of recent reports strongly indicated that chronic inflammation is closely linked to the development of liver cancer, implicating a theory of inflammation–fibrosis–cancer axis [1,2]. In Western countries, the most common cause of liver diseases is alcohol; by contrast, more than 90% of liver disease cases in Korea are due to viral infections and progressive hepatocellular carcinoma, which is often initiated by chronic hepatitis B virus (HBV) or hepatitis C virus infection and is considered a major worldwide health problem [3,4]. Approximately 350 million people worldwide are chronically infected by HBV. In Korea, in particular, 70% of liver cancer cases are caused by HBV infection, representing a serious national health issue.
- HBV contains four genes designated as S, C, P, and X. As with the rest, the X protein (HBx) encodes a 16.5- kDa regulatory protein including 154 amino acids. HBx enhances HBV replication and exerts multifunctional activities [5]. It acts as a transcriptional coactivator in the nucleus and stimulates various signal transduction pathways in the cytoplasm. Recently, HBx is known to have a kunitz domain, where it performs a special function as a serine protease inhibitor and transcriptional activatior [6].
- HBx does not bind directly to the DNA but regulates the activity of cellular proteins such as p53, BubR1, Pin1, UV-DDB, and HSP40/DanJ to bind directly or indirectly and contributes to liver cancer progression [7-10].
- The objective of this study was to develop an HBx purification process with elastin-like polypeptide (ELP) domains. ELPs are genetically engineered polypentapeptide biopolymers like mammalian elastin. They are composed of pentapeptide repeats of Val-Pro-Gly- Xaa-Gly, where the guest residue is any amino acid residue other than proline [11-14]. These polypeptides are soluble below their characteristic temperature transition and undergo an abrupt inverse temperature phase transition to aggregates upon heating and addition of salt.
- The hypothesis of this study was that a fusion protein constructed from ELP and HBx would result in HBx purification by ELP inverse phase-transitioning behavior. The ELP–HBx gene was designed, and the protein product was expressed in Escherichia coli. Results are presented for HBx purification. This study suggests that HBx can be purified in a rapid and simple manner using inverse transition cycling (ITC), and this method can be applied in determination of HBx 3D structures and HBx stability study.
1. Introduction
- 2.1. Cloning of full-length HBx gene
- To establish the HBx expression construct, polymerase chain reaction was performed with the inserting forward primer (5'-AAG CTT CTG GTT CCG CGT GGA TCC ATG GCT GCT AGG CTG TGC-3') and reverse primer (5'-AAG CTC AGC TAG GCA GAG GTG AAA AAG TTG CAT GG-3'). These resulting polymerase chain reaction fragments were digested with HindIII and BlpI, for the purpose of directional and in-frame ligation to the pQE-His-ELP vector, and inserted to pQE30-His-ELP (kindly provided by Dr. Yoon, Kyonggi University). Expression vectors containing the fusion gene were transformed into E coli BL21 (DE3) (Novagen, Madison, WI, USA) for protein overexpression.
- 2.2. Overexpression of recombinant fusion protein
- Fifty milliliters of LB and M9 minimum media with 100 μg/ml of ampicillin was inoculated with the expression strain and induced at a cell density of 0.6 (A600 nm) by the addition of 0.3 mM isopropylthiogalactoside (IPTG) at 3–4 hours.
- 2.3. Inclusion body purification
- Overexpressed cells were harvested by centrifugation (3200 × g for 15 minutes) and resuspended in 5 ml phosphate-buffered saline (PBS) with lysozme. Cells were lysed via sonication on ice and centrifuged (13,000 rpm for 15 minutes, 4 ℃) to harvest the inclusion body. The inclusion body pellet was washed (50 mM PBS, pH 7.4, containing 0.15 M NaCl and 1 mM EDTA) and centrifuged (15 minutes at 13,000 rpm at 4 ℃). The supernatant was decanted, and the precipitate was washed in 2 M urea for 20 minutes. Inclusion bodies were separated by centrifugation at 13,000 rpm for 30 minutes and washed in 0.5% Triton X-100 (v/v) and 10 mM EDTA (20 minutes incubation at room temperature). After washing, the inclusion bodies were recovered by centrifugation at 13,000 rpm for 15 minutes.
- 2.4. Inclusion body solubilisation and purification of recombinant ELP–HBx
- Washed inclusion bodies were dissolved in 0.1 M Tris–HCl, pH 8.6, containing 8 M urea for 24 hours at room temperature. Dissolved ELP–HBx fusion protein was separated by centrifugation at 13,000 rpm for 30 minutes, and the supernatant was collected. Then, denaturated fusion protein was dialyzed in 1 M urea solution. The recombinant ELP–HBx was purified by three rounds of ITC as described previously [13].
- 2.5. In vitro degradation of fusion protein
- Thrombin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in PBS to a concentration of 300 units/ml. Next, 3 μl of the enzyme solution was added to 100 μl of a purified ELP–HBx solution in PBS for a final activity of 0.3 units. The mixture was incubated overnight with gentle agitation at 37 ℃, after which sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described above.
- 2.6. Protein analysis
- Purified ELP–HBx fusion proteins were separated on a 12% SDS-PAGE gel and stained with silver. For Western blotting, ELP–HBx fusion proteins were separated and transferred onto a polyvinylidene difluoride membrane. The blot was stained with the appropriate Ab and developed using ECL as a substrate (GE Healthcare, Pittsburgh, USA).
2. Materials and Methods
- 3.1. Construction and optimum expression condition of ELP–HBx
- HBx encodes 16.5-kDa proteins which have 154 amino acids with different amino acid sequence between HBV subtypes. Figure 1 shows the HBx amino acid sequences in HBV subtypes and the ELP–HBx fusion protein sequences (Figures 1A and 1B). HBx was successfully cloned into the modified pQE30(+) expression vector, with subsequent adjacent insertion of ELP genes encoding ELP-(V5G3A2)120 (molecular weight, 50 kDa) at the protein N terminus (Figure 1C). HBx expressed following ELP proteins was induced by IPTG. HBx fusion proteins were successfully expressed by 0.3 mM IPTG in 3–4 hours in E coli BL21 (DE3) (Figure 2A).
- 3.2. Analysis of fusion protein
- The expression of ELP–HBx fusion proteins was checked by SDS-PAGE in soluble and cell debris part. In the results, the ELP–HBx fusion proteins were in the
- cell debris part. This indicates that ELP–HBx was expressed in the inclusion body (Figure 2B). ELP–HBx fusion protein was at 70-kDa fragments, which are comparable in terms of size and immunoreactivity to commercial his antibody (Figures 2B and 2C).
- 3.3. Purification of ELP–HBx fusion protein by ITC
- Under optimal expression conditions, 100 ml culture of IPTG induction was used for purification of ELP–HBx fusion proteins by ITC [13] (Figure 3A). ELP–HBx fusion protein was solubilized using 8 M urea solution, and the soluble protein was diluted to 2 M urea solution in a final concentration for protein purification. For purification, ELP-HBx fusion proteins were incubated for 10 minutes at 40 degree celcius in 1 M NaCl and were separated them by centrifugation at 13,000 rpm for 30 min. After centrifugation, the pellet was incubated in PBS at room temperature for 10 minutes to resolubilize ELP-HBx fusion proteins. SDSPAGE revealed the appropriate molecular weight bands for each protein, with minimal contamination, after three rounds of ITC (Figures 3B and 3C).
- 3.4. In vitro degradation of fusion protein
- The purified ELP–HBx fusion proteins were cleavaged for separation into ELP and HBx using thrombin.
- The fusion proteins were successfully digested by thrombin and separated into ELP and HBx (using SDSPAGE and Western blot), which are comparable in terms of size (16.5 kDa HBx) and immunoreactivity to the commercial HBx antibody (Figures 4A and 4B).
3. Results
Figure 1.

Hepatitis B virus X (HBx) amino acid sequences and construction of ELP–HBx. (A) Amino acid sequence alignment of HBx from HBV subtypes (adr, adw). (B) Amino acid sequence for ELP–HBx fusion protein. The fusion protein sequences indicate N-terminal 6xHis tag, polypentapeptide, and HBx (adr) proteins. (C) ELP–HBx fusion construction in modified pQE30 expression vector. ELP–HBx fusion proteins expressed adding isopropyl-thiogalactoside controlled T5 promoter.

Figure 2.
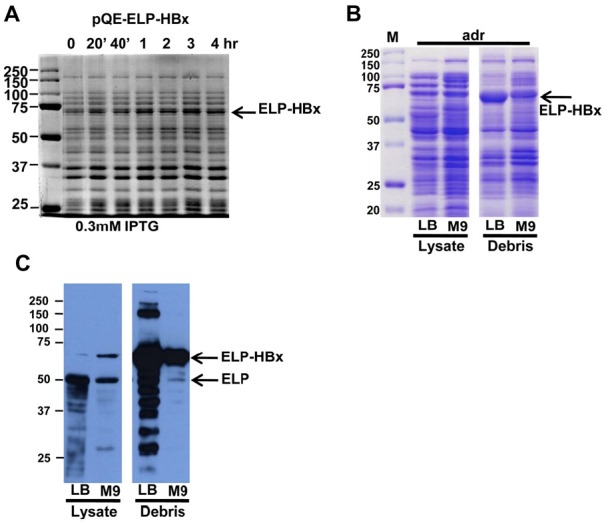
Expression condition of ELP–HBx fusion protein. (A) Expression of ELP–HBx protein depends on expression time in 0.3 M IPTG concentration. ELP–HBx in BL21 (DE3) strain was cultured until OD600 = 0.6 and induced by 0.3 M IPTG to express ELP–HBx recombinant proteins. Collection times were 20 min, 40 min, 1 h, 2 h, 3 h, and 4 h after adding IPTG. (B, C) Analysis of ELP–HBx fusion protein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis(SDS-PAGE) and Western blotting against anti-his antibody. Arrow indicates the ELP–HBx fusion proteins and ELP cleaved form.

- It has been shown previously that HBx protein is predominantly expressed in the insoluble inclusion body fraction of the bacterial cell lysate [15-17]. It also tends to form intracellular aggregates when expressed in insect cells using a baculovirus expression system [18]. The HBx protein has up to 52% of hydrophobic amino acids, and the protein contains four disulfide bonds in a unique arrangement [19]. These structure characteristics may increase HBx protein’s tendency toward intermolecular aggregation, improper folding, and accumulation in the inclusion-body fraction. To develop the process of purifying HBx protein, we used an ELP purification system to produce HBx protein in an easy and rapid manner. The protein purification system using ELP is a powerful system for the expression of HBx proteins in E coli because the ELP system has an ELP tag, which can aggregate/dissociate in inverse cycling at temperature transition of target proteins.
- A new protein and peptide purification method was developed that uses ELPs as the purification tag [13,20].The method of purifying proteins or peptides that are fused to a stimulus-responsive ELP retains this behavior in the complex milieu of contaminating cellular components [21]. This method regulates this phenomenon by adding salt to the fusion protein solution, and/or by heating the solution, the phase transition of the ELP fusion can be triggered in the cell lysate, resulting in the formation of micron-sized aggregates that are highly enriched in the ELP fusion, and that can easily be pelleted by centrifugation. Removing the supernatant, the pellet containing the fusion protein is in low ionic strength buffer. Following the resuspension step, insoluble E coli contaminants trapped in the fusion protein pellet are removed during centrifugation under conditions of low temperature and low salt in which the ELP fusion is soluble. One ITC lasts roughly 30 minutes, and pure protein is often obtained after three rounds of ITC, making this process extremely time-efficient and significantly advantageous compared to affinity purification [21]. This ELP purification process does not require any resins or specialized equipment such as a dedicated column.
- In this process, we also describe the required removal of the ELP tag after ELP–HBx purification. The ELP tag is simply removed after proteolytic cleavage, after which
- salt is added to trigger the ELP phase transition and the ELP tag is simply centrifuged out of the solution.
- In summary, this study suggests that HBx purification can be carried out by ELP inverse phase-transitioning behavior. The ELP–HBx fusion protein product was expressed in E coli, and HBx can be purified in a rapid and simple manner using ITC; this method can also be applied in determination of HBx 3D structures and HBx stability study.
4. Discussion
Figure 3.
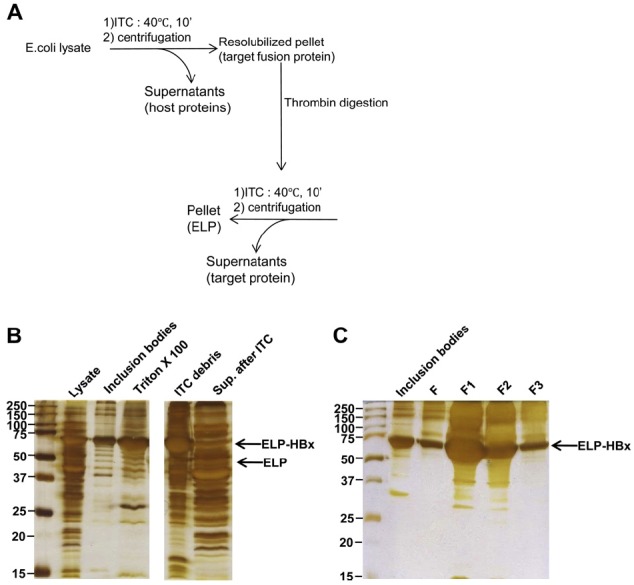
Inverse transition cycling(ITC) purification of ELP–HBx fusion proteins. (A) Diagram of the ITC method. Soluble ELP–HBx fusion protein from inclusion bodies in overexpressed Escherichia coli lysate was purified using 8 M Urea. For purification, ELP–HBx fusion proteins were incubated for 10 min at 40 ℃ in 1 M NaCl and then aggregated. After centrifugation, the resolubilized pellet was incubated at room temperature for 10 min in phosphate-buffered saline (PBS). The purified ELP–HBx fusion proteins were cleavaged for separation into ELP and HBx using thrombin. The fusion proteins were successfully digested by thrombin and separated into ELP and HBx using the ITC method. In the final step, the HBx target proteins are in supernatant. (B) ITC purification of ELP–HBx target proteins. Lysate = total lysate; inclusion bodies and TritonX100: = purification of inclusion bodies from total lysate; ITC debris = pellet after ITC; Sup.after ITC = supernatant after ITC purification. (C) Purified target proteins. F to F3 are fraction numbers of ELP–HBx fusion proteins after ITC purification.

Figure 4.
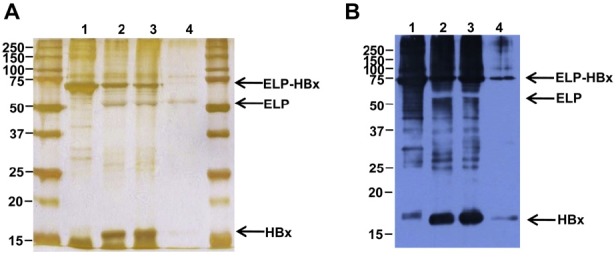
Thrombin cleavage of purified ELP–HBx fusion proteins. (A) Thrombin cleavage of ELP–HBx fusion proteins by silver staining after ITC purification and (B) Western blot analysis against anti-HBx antibody. Arrow indicates ELP–HBx fusion proteins, cleaved ELP proteins, and HBx proteins. 1 = purified ELP–HBx; 2, 3 = thrombin treated ELP–HBx; 4 = purified HBx after thrombin treatment.

-
Acknowledgements
- This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2009-351-2009-2-C00131).
- 1. Maeda S Kamata H Luo JL et al.. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 7;2005;121(7). 977−90. PMID: 15989949.ArticlePubMed
- 2. Murata H Kawano S Tsuji S et al.. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol 2;1999;94(4). 451−5. PMID: 10022645.ArticlePubMed
- 3. Cougot D Neuveut C Buendia MA . HBV induced carcinogenesis. J Clin Virol 12;2005;34(Suppl 1). S75−8. PMID: 16461228.Article
- 4. Sherman M . Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis 2005;25(2). 143−54. PMID: 15918143.ArticlePubMed
- 5. Bouchard MJ Schneider RJ. . The enigmatic X gene of hepatitis B virus. J Virol. 12;2004;78(23). 12725−34. PMID: 15542625.ArticlePubMedPMC
- 6. Kanda T Yokosuka O Imazeki F et al.. Hepatitis B virus X protein (HBx)-induced apoptosis in HuH-7 cells: influence of HBV genotype and basal core promoter mutations. Scand J Gastroenterol 5;2004;39(5). 478−85. PMID: 15180187.ArticlePubMed
- 7. Bontron S Lin-Marq N . Hepatitis B virus X protein associated with UV-DDB1 induces cell death in the nucleus and is functionally antagonized by UV-DDB2. J Biol Chem. 10;2002;277(41). 38847−54. PMID: 12151405.ArticlePubMed
- 8. Datta S Banerjee A Chandra PK et al.. Pin1–HBx interaction: a step toward understanding the significance of hepatitis B virus genotypes in hepatocarcinogenesis. Gastroenterology 8;2007;133(2). 727−8. author reply 728-9.PMID: 17681194.ArticlePubMed
- 9. Kim S Park SY Yong H et al.. HBV X protein targets hBubR1, which induces dysregulation of the mitotic checkpoint. Oncogene 5;2008;27(24). 3457−64. PMID: 18193091.ArticlePubMed
- 10. Yoo YG Oh SH Park ES et al. Hepatitis B virus X protein enhances transcriptional activity of hypoxia-inducible factor- 1alpha through activation of mitogen-activated protein kinase pathway. J Biol Chem. 3 10 2003;278(40). 39076−84. PMID: 12855680.ArticlePubMed
- 11. Banki MR Feng L Wood DW . Simple bioseparations using selfcleaving elastin-like polypeptide tags. Nat Methods 9;2005;2(9). 659−61. PMID: 16074986.ArticlePubMed
- 12. Fong BA Wu WY Wood DW . Optimization of ELP-intein mediated protein purification by salt substitution. Protein Expr Purif 8;2009;66(2). 198−202. PMID: 19345265.ArticlePubMed
- 13. Meyer DE Chilkoti A . Purification of recombinant proteins by fusion with thermally-responsive polypeptides. Nat Biotechnol 11;1999;17(11). 1112−5. PMID: 10545920.ArticlePubMed
- 14. Wu WY Mee C Califano F et al.. Recombinant protein purification by self-cleaving aggregation tag. Nat Protoc 2006;1(5). 2257−62. PMID: 17406465.ArticlePubMed
- 15. Jameel S Siddiqui A Maguire HF Rao KV . Hepatitis B virus X protein produced in Escherichia coli is biologically functional. J Virol 8;1990;64(8). 3963−6. PMID: 2196386.ArticlePubMedPMC
- 16. Marczinovits I Somogyi C Patthy A et al.. An alternative purification protocol for producing hepatitis B virus X antigen on a preparative scale in Escherichia coli. J Biotechnol 8;1997;56(2). 81−8. PMID: 9304871.ArticlePubMed
- 17. Rui E de Moura PR Kobarg J. . Expression of deletion mutants of the hepatitis B virus protein HBx in E. coli and characterization of their RNA binding activities. Virus Res 4;2001;41(1-2). 59−73. PMID: 11226575.Article
- 18. Urban S Hildt E Eckerskorn C et al. Isolation and molecular characterization of hepatitis B virus X-protein from a baculovirus expression system. Hepatology 10;1997;26(4). 1045−53. PMID: 9328333.ArticlePubMed
- 19. Gupta A Mal TK Jayasuryan N Chauhan VS . Assignment of disulphide bonds in the X protein (HBx) of hepatitis B virus. Biochem Biophys Res Commun 7;1995;212(3). 919−24. PMID: 7626131.ArticlePubMed
- 20. Trabbic-Carlson K Liu L Kim B Chilkoti A . Expression and purification of recombinant proteins from Escherichia coli: comparison of an elastin-like polypeptide fusion with an oligohistidine fusion. Protein Sci. 12;2004;13(12). 3274−84. PMID: 15557268.ArticlePubMedPMC
- 21. Hassouneh W Christensen T Chilkoti A . Elastin-like polypeptides as a purification tag for recombinant proteins. Curr Protoc Protein Sci 2010;Aug. CHAPTER: Unit 6.11.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Evaluation of machine learning algorithms to predict the hydrodynamic radii and transition temperatures of chemo-biologically synthesized copolymers
Jared S. Cobb, Maria A. Seale, Amol V. Janorkar
Computers in Biology and Medicine.2021; 128: 104134. CrossRef - Machine learning to determine optimal conditions for controlling the size of elastin-based particles
Jared S. Cobb, Alexandra Engel, Maria A. Seale, Amol V. Janorkar
Scientific Reports.2021;[Epub] CrossRef - Influence of miR‐520e‐mediated MAPK signalling pathway on HBV replication and regulation of hepatocellular carcinoma cells via targeting EphA2
Jing‐hui Tian, Wen‐dong Liu, Zhi‐yong Zhang, Li‐hua Tang, Dong Li, Zhao‐ju Tian, Shao‐wei Lin, Ying‐jie Li
Journal of Viral Hepatitis.2019; 26(4): 496. CrossRef - Elastin‐like polypeptides: A strategic fusion partner for biologics
Agnes Yeboah, Rick I. Cohen, Charles Rabolli, Martin L. Yarmush, Francois Berthiaume
Biotechnology and Bioengineering.2016; 113(8): 1617. CrossRef - Elastin-like polypeptides as a promising family of genetically-engineered protein based polymers
Tomasz Kowalczyk, Katarzyna Hnatuszko-Konka, Aneta Gerszberg, Andrzej K. Kononowicz
World Journal of Microbiology and Biotechnology.2014; 30(8): 2141. CrossRef


 PubReader
PubReader Cite
Cite
