Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 5(Suppl); 2014 > Article
-
Original Article
The Usefulness of the Tuberculosis Skin Test and the Interferon-gamma Release Assay in the Diagnosis of Latent Tuberculosis Infection in South Korea - Ju Young Jang, In Won Park, Byoung Whui Choi, Jae Chol Choi
-
Osong Public Health and Research Perspectives 2014;5(Suppl):S18-S23.
DOI: https://doi.org/10.1016/j.phrp.2014.10.009
Published online: November 12, 2014
Division of Pulmonary Medicine, Department of Internal Medicine, Chung-Ang University School of Medicine, Seoul, Korea
- ∗Corresponding author. medics27@cau.ac.kr
• Received: October 15, 2014 • Revised: October 26, 2014 • Accepted: October 27, 2014
© 2014 Published by Elsevier B.V. on behalf of Korea Centers for Disease Control and Prevention.
This is an Open Access article distributed under the terms of the CC-BY-NC License (http://creativecommons.org/licenses/by-nc/3.0).
Abstract
-
Objectives
- South Koreans receive the bacillus Calmette-Guerin (BCG) vaccination, which influence the result of the tuberculin skin test (TST); however, only a few studies have described the usefulness of the TST and interferon-γ release assay (IGRA) for diagnosing latent TB infection (LTBI). Therefore, our aim was to determine the usefulness of the TST and IGRA for diagnosing LTBI in a household contacts investigation.
-
Methods
- We reviewed the 329 household contacts who visited Chung-Ang University Hospital (Seoul, Korea) from May 1, 2011 to February 28, 2014. To evaluate the effectiveness of TST and IGRA for the diagnosis of LTBI, we examined the concordance rate between the two tests, based on age. We also evaluated the risk factors for LTBI.
-
Results
- The concordance rate between the two tests in individuals 0–24 years, 25–54 years, and over 55 years were 82.6% (κ = 0.64, p < 0.01), 68.9% (κ = 0.40, p < 0.01), and 68.4% (κ = 0.35, p < 0.01), respectively. The ratio of positive TST to negative IGRA was higher in individuals 25–44 years old, whereas the ratio of negative TST to positive IGRA was higher in individuals older than 55 years old. Based on the TST, the risk factor for LTBI was a cavity (p < 0.01). When using IGRA, the risk factors were contact time (p = 0.04) and age over 55 years old (p = 0.02).
-
Conclusion
- The concordance rate between TST and IGRA was not good after the age of 25 years. The IGRA test reflects the known risk factors more exactly.
- Tuberculosis (TB) remains a major public health problem in the world, especially in low- and middle-income countries. According to the World Health Organization, in 2011, there were 8.7 million new incident TB cases and 0.99 million TB deaths (just under 0.43 million human immunodeficiency virus-associated TB deaths) [1]. Tuberculosis is also a major public problem in South Korea because the incidence and mortality remain high [2]. Therefore, it is important to reduce the TB incidence by continuous tuberculosis supervising work.
- The first priority in a TB prevention and control program is the identification and treatment of all people with active TB; the second priority is contact investigation to find people who are exposed to TB patients [3, 4] In South Korea since 1995, the diagnosis and treatment of latent TB infection (LTBI) in high-risk patients has been added in TB supervising work. After 2011, tuberculosis management guidelines have recommended diagnosing and treating contact patients and active TB patients. The tuberculin skin test (TST) and interferon-γ release assay (IGRA) are tests used in diagnosing LTBI. Because the TST is affected by the bacillus Calmette-Guerin (BCG) vaccination, the TST has low specificity in the BCG vaccination population. The IGRA, which measures in vitro interferon-γ production by T cells sensitized with the Mycobacterium tuberculosis antigens ESAT-6 and CFP-10, was created in 1995 and was put into practical use in 2000 [5,6] The IGRA offers better specificity (98–100%) and good sensitivity (79–97%); this test is unaffected by the BCG vaccination [7]. Therefore, IGRA is recommended for the BCG vaccination population [8–10]. South Koreans receive BCG vaccination in infancy. Therefore, the effectiveness of TST, despite its simplicity, is being questioned in South Korea. However, few studies have compared the usefulness of TST and IGRA for TB diagnosis in South Korea. In this study, we aimed to compare the positive rate of TST and IGRA in a contact investigation and to determine the risk factors for LTBI.
Introduction
- 2.1 Study design and participants
- We performed a retrospective study at the Chung-Ang University Hospital (Seoul, Korea) between May 1, 2011 and February 28, 2014. The study participants included all patients with active TB and a household contact population during these periods. We collected demographic information, clinical data, acid-fast bacillus (AFB) sputum smears, TB cultures, the presence of cavitation on chest X-ray, TB-polymerase chain reaction, and contact time. To compare the effectiveness of TST and IGRA, we examined the positive rate and concordance rate, based on age. We also analyzed the risk factors of TST and IGRA.
- 2.2 Diagnosis of LTBI and active TB
- Chest X-ray, IGRA, and TST were conducted on contact patients. The TST was performed on the forearm using a 2-tuberculin unit (TU) dose of purified protein derivative RT23 (0.1 mL). Indurations were measured after 48–72 hours. We defined a positive test result for an induration diameter of ≥10 mm. The interferon-γ release assay was performed using the QFT-TB Gold-In-Tube test (QFT-GIT; Cellestis Ltd, Carnegie, VIC, Australia) in accordance with the manufacturer's instructions. The results of IGRA were interpreted as positive in ≥0.35 IU/mL [11] Active TB was diagnosed, based on the presence of M. tuberculosis in culture, AFB in the microscopic examination of the clinical specimen, or chest X-ray suggestive of TB with response to antituberculosis medications [12].
- 2.3 Statistical analysis
- Statistical analysis was performed using SPSS version 18 (SPSS Inc., Chicago, IL, USA). The data were presented as the mean value with standard deviation. The positive rate and risk factors between TST and IGRA based on age was compared using the Chi-square test. The concordance rate between the two tests was evaluated using the κ coefficient. A value of p < 0.05 was considered statistically significant.
Materials and methods
- From May 1, 2011 to February 28, 2014, 157 index cases had 329 household contacts, and the contacts performed contact investigation. Four (1.21%) contacts were diagnosed as having active TB. One contact had a positive culture and the remaining three contacts had negative cultures. The median age of the close contacts was 37.7 ± 22.2 years. More than one-half (59.6%) of the patients were female and 12 (3.8%) patients were diabetic. The positive rate of TST and IGRA were 46.4% and 45.9%, respectively. Among the 157 index patients, 43 (27.4%) patients had positive smears, 118 (75.2%) patients had positive cultures, and 22 (14.0%) patients had a cavity on their chest X-ray (Table 1).
- 3.1 Positive rate of TST and IGRA, according to age
- The positive rate of TST of patients 25–34 years and 35–44 years was 46.3% and 63.8%, respectively. The positive rate of IGRA in patients 25–34 years and 35–44 years was 21.4% and 47.8%, respectively. In the p for trend value, the positive rate of TST did not tend to increase with increasing age. However, IGRA results tended to increase significantly with increasing age (p < 0.01; Figure 1).
- 3.2 Concordance rate between TST and IGRA, according to age
- Among 329 patients, 218 household contact patients underwent the TST and IGRA together. Among this population, the concordance rate was 70.2% (153/218). The concordance rate between the two tests in patients aged 0–24 years, 25–54 years, and over 55 years was 82.6%, 68.9%, and 68.4%, respectively. The κ value between the two tests was 0.638 (p < 0.01) in the 0–24 years age group, 0.404 (p < 0.01) in the 25–54 years age group, and 0.350 (p < 0.01) in the >55 years age group (Table 2). The ratio of the TST+/IGRA− group was higher (26.0%) in the 25–54 years age group than in the other age groups. In patients aged >55 years, the ratio of the TST+/IGRA− group was decreased substantially (11.8%) and the ratio of the TST−/IGRA + group was increased (19.7%).
- 3.3 Risk factors of TST and IGRA positivity in household contacts
- To see the diagnostic usefulness of TST and IGRA, we assessed the relationship between the risk factors of latent tuberculosis and these examinations. Among the 329 patients, the TST was performed in 312 contact patients. When we used the TST as the diagnostic test for LTBI in an unadjusted analysis, the risk factors for LTBI were the presence of a lung cavity [odds ratio (OR) = 1.96, p = 0.04]. In multivariate analysis, the risk factor for TST positivity was the presence of a lung cavity (OR = 3.81, p < 0.01; Table 3). Among the 329 patients, IGRA was performed in 235 contact patients. When we used the IGRA as the diagnostic test for LTBI in an unadjusted analysis, the risk factors for LTBI were age over 55 years (OR = 3.00, p < 0.01), positive smear results (OR = 1.78, p = 0.04), and contact time of >10 hours per day (OR = 2.13, p = 0.02; Table 4). In multivariate analysis, the risk factors for IGRA positivity were age over 55 years (OR = 2.06, p = 0.02) and contact time of >10 hours (OR = 2.06, p = 0.04). Smear positivity also showed a trend [OR = 1.86; confidence interval (CI), 0.98–3.53; p = 0.06], but it did not reach the statistical difference (Table 4).
- On observing the positive rate of TST and IGRA by contact time, the positive rate of IGRA tended to increase significantly by contact time, (p = 0.04). However, the TST did not show any tendency (Table 5).
Results
- A contact investigation is the most effective method when supervising pulmonary tuberculosis in countries with a low incidence of tuberculosis [13,14] In the past, the TST has been used for the diagnosis of LTBI. However, in many countries, IGRA is being introduced as a standard method because it has high specificity and good predictive value in the development of active TB. [15,16] In the United States of America, IGRA can be used as an alternative to TST (i.e., one-step strategy) [17,18] In England and Norway, when the TST is positive, IGRA is recommended for diagnosing latent tuberculosis (i.e., 2-step strategy) [19] In South Korea, the TST is the basic method in diagnosing LTBI. However, the TST/IGRA combined method or IGRA-only method are allowed. However, it is important to consider a false-positive response when using TST because BCG vaccination can influence the results of TST.
- Our study showed a moderate concordance rate between TST and IGRA, which was 70.18% (153/218). However, when we compared the concordance rate according to age, the concordance was different. The κ value showed good concordance rate in individuals aged 0–24 years. However, the concordance rates decreased with increasing age. In particular, the frequency of the TST+/IGRA− group is higher in the 25–54 years age group than in the other groups. In addition, the TST positive rate increased between 25 years and 54 years but decreased after 55 years. However, the IGRA positivity rate significantly increased with age.
- The TST positive rate increases significantly with age [20]. This is because old patients have more chances to become infected during their lifetime in TB-endemic areas. In addition, they are more vulnerable to TB infection because of their decreased immunity. This result is consistent with the IGRA positive rate in our study. However, the TST positive rate did not show this trend in this study. This can be explained by BCG vaccination. Individuals who had BCG vaccination have higher false-positive results with the TST [21,22]. However, some research suggests that the false-positive effect decreases 5–10 years after BCG vaccination [23] However, if individuals received boosting vaccination, the effect can last up to 20 years [24]. In South Korea, BCG revaccination at 12 years old was recommended until 1997. After 1997, BCG vaccination has only been recommended in infancy. We did not confirm the boosting vaccination status in these populations, although individuals over the age of 25 years probably received BCG revaccination at the age of 12 years because it was a national guideline. Therefore, the 25–54 years age group may have a higher false-positive reaction in the TST.
- The risk factors of tuberculosis infection have been evaluated in several studies [25–27]. In our study, TST positivity was associated with a cavity, and IGRA positivity was associated with an age over 55 years and a contact time of >10 hours per day. A positive result of the AFB smear examination of patients with TB has been associated with TB infection among contacts. Our study also showed that the result of the sputum examination was associated with LTBI when we used IGRA as the diagnostic method in an unadjusted analysis. We failed to reach the statistical difference in the multivariate analysis, although the OR was similar in adjusted analysis. Therefore, if the sample size increased, a statistical difference may be observed.
- The length of exposure to index cases is also an important factor for LTBI. We found it was associated with LTBI when we used IGRA. The positive rate was increased by the contact time when we used IGRA as the diagnostic method for diagnosing LTBI. However, when we used the TST, it was not a risk factor for LTBI. A positive reaction to TST or IGRA does not represent recent infection. South Korea is an intermediate TB burden country. Therefore, some people have been infected previously. It could be more prominent in older populations. There is no method to differentiate between a recent infection and a previous infection. Therefore, our evaluation of the risk factors for TB infection could have some bias. However, our results showed that IGRA is more consistent, compared to the results of previous studies. Therefore, in South Korea, IGRA may be more valuable for contact investigation of people over the age of 25 years.
- Four persons among the 329 contacts were diagnosed as having active TB during the contact investigation. It is difficult to decide that active TB originated from the index cases, but the incidence (1215 per 100,000) is higher than the incidence of active TB in South Korea (which is 100 per 100,000). In addition, three patients among four active TB patients had no symptoms. Early diagnosis of active TB is an important tool to control TB. Therefore, chest X-ray is an important tool in contact investigations in TB-endemic areas.
- Our study has several limitations. First, all contacts were not screened by the two tests together because of the study's retrospective design. Therefore, this could cause some selection bias. Second, because of the retrospective study design, all information was not gathered from the contacts. We also could not check the patients' BCG vaccination status. However, the BCG vaccination program followed the national guideline. Therefore, the possibility of no vaccination could be low. Finally, the small sample size made it difficult to obtain the statistical difference. Therefore, a large group prospective study is needed.
- In conclusion, the concordance rate between TST and IGRA in patients over the age of 25 years is not good (Table 5). The TST+/IGRA− rate was high among patients aged 25–54 years. In addition, the positive result of IGRA was associated with contact time, old age, and smear positivity. Therefore, the possibility of false-positive reaction on TST must be considered in people who received a boosting BCG vaccination.
Discussion
- None of the authors has any conflicts of interest to declare.
Conflicts of interest
-
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Article information
- 1. World Health Organization (WHO) . WHO report: global tuberculosis control. 2011. WHO; Geneva, Switzerland: Available from:. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. [accessed 09.11.14].
- 2. World Health Organization (WHO) . Global tuberculosis report 2012. 2012. WHO; Geneva, Switzerland: Available from:. http://www.who.int/tb/publications/global_report/2011/gtbr11_full.pdf. [accessed 09.11.14].
- 3. Jasmer R.M., Nahid P., Hopewell P.C.. Clinical practice. Latent tuberculosis infection. N Engl J Med 347(23). 2002 Dec;1860−1866. PMID: 12466511.ArticlePubMed
- 4. Marks S.M., Taylor Z., Qualls N.L.. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med 162(6). 2000 Dec;2033−2038. PMID: 11112109.ArticlePubMed
- 5. Fong K.S., Tomford J.W., Teixeira L.. Challenges of interferon-gamma release assay conversions in serial testing of health-care workers in a TB control program. Chest 142(1). 2012 Jul;55−62. PMID: 22796839.ArticlePubMed
- 6. Ringshausen F.C., Schablon A., Nienhaus A.. Interferon-gamma release assay for the tuberculosis serial testing of health care workers: a systematic review. J Occup Med Toxicol 7(1). 2012 Jun;6PMID: 22537915.ArticlePubMed
- 7. Murthy S., Adlakha A., Allan K.. What is the impact of switching from skin tests to blood IGRA for latent TB infection screening in an occupational health setting? Am J Respir Crit Care Med 185:2012;A4729. Article
- 8. Haldar P., Thuraisingam H., Patel H.. Single-step QuantiFERON screening of adult contacts: a prospective cohort study of tuberculosis risk. Thorax 68(3). 2013 Mar;240−246. PMID: 22956558.ArticlePubMed
- 9. Rangaka M.X., Wilkinson K.A., Glynn J.R.. Predictive value of interferon-gamma release assays for incident active tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 12(1). 2012 Aug;45−55. PMID: 21846592.ArticlePubMed
- 10. Zwerling A., Behr M.A., Verma A.. The BCG World Atlas: a database of global BCG vaccination policies and practices. PLoS medicine 8(3). 2011 Mar;e1001012PMID: 21445325.ArticlePubMed
- 11. Shim T.S.. Diagnosis and treatment of latent tuberculosis infection. Korean J Med 82(3). 2012;284−290.Article
- 12. Joint Committee for the Development of Korean Guidelines for Tuberculosis. Korean guidelines for tuberculosis. 1st ed.2011. Korea Centers for Disease Control and Prevention; Cheongwon, Korea.
- 13. Hong Y.P., Kim S.J., Lew W.J.. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis 2(1). 1998 Mar;27−36. PMID: 9562108.PubMed
- 14. Wang L., Turner M.O., Elwood R.K.. A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 57(9). 2002 Sep;804−809. PMID: 12200526.ArticlePubMed
- 15. Pai M., Zwerling A., Menzies D.. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med 149(3). 2008 Aug;177−184. PMID: 18593687.ArticlePubMed
- 16. Menzies D., Pai M., Comstock G.. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med 146(5). 2007 Mar;340−354. PMID: 17339619.ArticlePubMed
- 17. Jensen P.A., Lambert L.A., Iademarco M.F.; Centers for Disease Control and Prevention (CDC). Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep 54(RR-17). 2005;1−141. PMID: 16382216.
- 18. Mazurek G.H., Jereb J., Vernon A.; IGRA Expert Committee; Centers for Disease Control and Prevention (CDC). Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recomm Rep 59(RR-5). 2010;1−25. PMID: 20577159.
- 19. National Collaborating Centre for Chronic Conditions (UK); Centre for Clinical Practice at NICE (UK). National Institute for Health and Clinical Excellence: guidance. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. 2011. National Institute for Health and Clinical Excellence; London, UK.
- 20. Aissa K., Madhi F., Ronsin N.; CG94 Study Group. Evaluation of a model for efficient screening of tuberculosis contact subjects. Am J Respir Crit Care Med 177(9). 2008 May;1041−1047. PMID: 18263798.ArticlePubMed
- 21. Joos T.J., Miller W.C., Murdoch D.M.. Tuberculin reactivity in Bacille Calmette-Guerin vaccinated populations: a compilation of international data. Int J Tuberc Lung Dis 10(8). 2006 Aug;883−891. PMID: 16898373.PubMed
- 22. Sterne J.A., Rodrigues L.C., Guedes I.N.. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis 2(3). 1998 Mar;200−207. PMID: 9526191.PubMed
- 23. Farhat M., Greenaway C., Pai M.. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 10(11). 2006 Nov;1192−1204. PMID: 17131776.PubMed
- 24. Barreto M.L., Cunha S.S., Pereira S.M.. Neonatal BCG protection against tuberculosis lasts for 20 years in Brazil. Int J Tuberc Lung Dis 9(10). 2005 Oct;1171−1173. PMID: 16229231.PubMed
- 25. Gran G., Assmus J., Dyrhol-Riise A.M.. Screening for latent tuberculosis in Norwegian health care workers: high frequency of discordant tuberculin skin test positive and interferon-gamma release assay negative results. BMC Public Health 13(1). 2013 Apr;353PMID: 23590619.ArticlePubMed
- 26. Nienhaus A., Loddenkemper R., Hauer B.. Latent tuberculosis infection in healthcare workers—evaluation of an interferon-gamma release assay. Pneumologie 61(4). 2007 Apr;219−223. [in German]. PMID: 17455134.ArticlePubMed
- 27. Storla D.G., Kristiansen I., Oftung F.. Use of interferon gamma-based assay to diagnose tuberculosis infection in health care workers after short term exposure. BMC Infect Dis 9:2009 May;60PMID: 19432995.ArticlePubMed
References
Figure 1Positive rate of the tuberculin skin test and interferon-γ release assay, according to age group. IGRA = interferon-γ release assay; TST = tuberculin skin test.
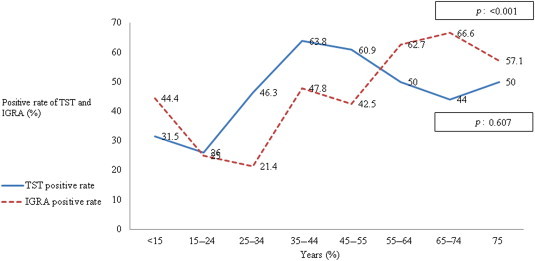

Table 1Baseline characteristics of the contacts and the index cases.
Table 2The concordance rate of the tuberculin skin test and interferon-γ release assay, according to age group (n = 218).
Table 3Risk factors for latent tuberculosis infection using tuberculin skin test as the diagnostic method (n = 312).
| TST positive (n = 145, 46.5%) | Unadjusted OR (95% CI), p* | Adjusted OR (95% CI), p* | |
|---|---|---|---|
| Contact characteristics | |||
| Male | 63/145 (43.4) | 0.83 (0.53–1.30), 0.42 | |
| Age (y) | 41.2 ± 20.3 | ||
| Age over 55 y | 42/145 (28.9) | 1.11 (0.67–1.82), 0.69 | 1.43 (0.81–2.53), 0.22 |
| Contact time (>10 h/d)a | 58/109 (53.2) | 0.99 (0.56–1.742), 0.97 | 1.05 (0.59–1.87), 0.88 |
| Diabetesa | 7/133 (5.3) | 1.72 (0.53–5.55), 0.35 | |
| Index characteristics | n = 145 (46.5) | ||
| Smear positive | 48/145 (33.1) | 1.38 (0.85–2.25), 0.19 | 1.43 (0.81–2.53), 0.22 |
| Liquid culture positive | 112/145 (77.2) | 1.03 (0.61–1.75), 0.90 | |
| Solid culture positive | 104/145 (71.7) | 1.24 (0.76–2.02), 0.37 | |
| Cavity | 25/145 (17.2) | 1.96 (0.10–3.84), 0.04 | 3.81 (1.61–9.02), <0.01 |
Table 4Risk factors for latent tuberculosis infection using interferon-gamma release assay as the diagnostic method (n = 235).
| IGRA positive (n = 108, 46.0%) | Unadjusted OR (95% CI), p* | Adjusted OR (95% CI), p* | |
|---|---|---|---|
| Contact cases | |||
| Male | 39/108 (36.1) | 1.18 (0.69–2.01), 0.52 | |
| Age (y) | 50.7 ± 16.1 | ||
| Age > 55 y | 51/108 (47.2) | 3.00 (1.72–5.24), <0.01 | 2.06 (1.10–3.84), 0.02 |
| Contact time (>10 h/d)a | 44/87 (50.6) | 2.13 (1.10–4.12), 0.02 | 2.06 (1.05–4.05), 0.04 |
| Diabetesa | 5/97 (5.2) | 1.03 (0.30–3.49), 0.95 | |
| Index cases | n = 108 (45.9) | ||
| Smear positive | 40/108 (37.0) | 1.78 (1.01–3.13), 0.04 | 1.86 (0.98–3.53), 0.06 |
| Liquid culture positive | 86/108 (79.6) | 1.28 (0.69–2.39), 0.42 | |
| Solid culture positive | 81/108 (75.0) | 1.41 (0.79–2.51), 0.23 | |
| Cavity | 13/108 (12.0) | 0.77 (0.36–1.65), 0.51 | 1.11 (0.45–2.71), 1.11 |
Table 5The positive rate of tuberculin skin test and interferon-gamma release assay, according to contact time.
Figure & Data
References
Citations
Citations to this article as recorded by 

- Household tuberculosis contact investigation in a tuberculosis-prevalent country
Jung Seop Eom, Insu Kim, Won-Young Kim, Eun-Jung Jo, Jeongha Mok, Mi-Hyun Kim, Kwangha Lee, Ki Uk Kim, Hye-Kyung Park, Min Ki Lee
Medicine.2018; 97(3): e9681. CrossRef - QuantiFERON-TB Gold In-tube test for the diagnosis of active and latent tuberculosis in selected health facilities of Addis Ababa, Ethiopia
Selam Niguse, Kassu Desta, Gebremdihin Gebremichael, Atsebeha Gebrezgeaxier, Mulluwork Getahun, Desta Kassa
BMC Research Notes.2018;[Epub] CrossRef - Predictors for false-negative QuantiFERON-TB Gold assay results in patients with extrapulmonary tuberculosis
Youn Jeong Kim, Ji Young Kang, Sang Il Kim, Mee Soo Chang, Yang Ree Kim, Yeon Joon Park
BMC Infectious Diseases.2018;[Epub] CrossRef


 PubReader
PubReader Cite
Cite
