Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 6(6); 2015 > Article
-
Original Article
Assessment of Antisecretory, Gastroprotective, andIn-vitro Antacid Potential ofDaucus carota in Experimental Rats - Phool Chandraa, Kamal Kishoreb, Ashoke Kumar Ghosha
-
Osong Public Health and Research Perspectives 2015;6(6):329-335.
DOI: https://doi.org/10.1016/j.phrp.2015.10.006
Published online: October 26, 2015
aSchool of Pharmaceutical Sciences, IFTM University, Lodhipur Rajput, Moradabad, India
bDepartment of Pharmacy, MJP Rohilkhand University, Bareilly, India
- ∗Corresponding author. chandraphool@yahoo.co.in
Copyright © 2015 Korea Centers for Disease Control and Prevention. Published by Elsevier Korea LLC. All rights reserved.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- In Indo China, carrots have been reported to regulate the functions of the stomach and intestines. The objective of the present investigation was to unravel the therapeutic potential of 50% ethanol extract from Daucus carota roots (EDC) on antisecretory, gastroprotective, and in vitro antacid capacity using experimental rats.
-
Methods
- Assessment of EDC antisecretory and in vivo antacid capacities was carried out using a pyloric ligation induced ulcer model. The gastroprotective effect was assessed with an absolute ethanol induced ulcer model. The integrity of gastric mucosa was evaluated using the estimation of glutathione and gastric mucus level and with histopathological examination of gastric mucosal cells. The in-vitro antacid capacity was evaluated using a titration method. The effect of the extract on the liver was assessed by measuring serum biochemical parameters.
-
Results
- The EDC significantly (p < 0.01–0.001) reduced gastric lesions in both models. Furthermore, the EDC also significantly (p < 0.05–0.001) reduced the volume of gastric content whereas the total acidity was significantly (p < 0.05–0.001) reduced with the doses of 100 mg/kg and 200 mg/kg EDC. Moreover, the mucus content and glutathione level increased significantly (p < 0.05) in the absolute alcohol-induced ulcer. The EDC also showed in-vitro antacid capacity. Histopathological studies further confirmed the potential of EDC by inhibiting congestion, edema, hemorrhage, and necrosis in gastric mucosa.
-
Conclusion
- The EDC exerted antisecretory, gastroprotective, and in vitro antacid potential. These activities could be attributed due to the presence of glycosides, phenolics, tannins, alkaloids, and flavonoids.
- Gastric ulcers are one of the major gastrointestinal disorders that occur due to an imbalance between the offensive (gastric acid secretion) and defensive (gastric mucosal integrity) factors [1]. Nowadays, there are two main approaches for curing peptic ulcers: the first approach is to reduce the gastric acid secretion and another approach is to reinforce the gastric mucosal protection [2].
- Plants have been a valuable foundation of new drugs and considered as an alternative strategy in search for new drugs. There is a rich profusion of plants used in traditional medicine known to possess antiulcer properties [3]. Daucus carota L. ssp. sativus (family: Apiaceae) is an annual or biannual herb mostly confined to the temperate regions of Europe, Asia, and Africa [4]. In Indo China, carrots are used to regulate the functions of the stomach and intestines [5]. Pharmacologically, scientists have shown that the extract obtained from the Daucus carota possesses analgesic, anti-inflammatory [6], antifertility [7], antitumor [8], hepatoprotective [9] and hypoglycemic properties [10]. Patil et al [11] studied the anti-inflammatory effects on experimental colitis in rats. Extracts of umbels of Daucus carota L. ssp. carota were evaluated against ethanol induced gastric ulcer in rats [12]. Roots contain pyrrolidine, daucine [13], vitamin A, daucosterol, thiamine, riboflavin, nicotinic acid, vitamin C (in the form of protein-ascorbic acid complex), and vitamin D [14].
- On the basis of literature review, the objective of the present investigation was to unravel the therapeutic potential of 50% ethanol extract from Daucus carota roots (EDC) antisecretory, gastroprotective, and in-vitro antacid capacity using experimental rats.
Introduction
- 2.1 Collection of plant material
- The fresh roots of Daucus carota L ssp. sativus were collected from the Bazikhera of Unnao district belonging to Uttar Pradesh. The plant materials were taxonomically identified and authenticated by Dr D.C. Saini, Scientist E at Birbal Sahni Institute of Palaeobotony, Lucknow with reference no. 13597.
- 2.2 Preparation of the extract
- The fresh roots (1 kg) of D. carota were peeled, washed, cut into small pieces, and homogenized in a blender without adding water. They were first defatted with petroleum ether and then extracted with 50% ethanol using a soxhlet extractor. The ethanol extract was filtered and the filtrate was dried in a rota evaporator to yield 20.23% w/w. EDC roots were stored in a desiccator for further preliminary phytochemical screening and pharmacological evaluation.
- 2.3 Preliminary phytochemical studies
- The extract obtained was subjected to preliminary qualitative tests for various plant constituents using suitable chemical tests 15, 16.
- 2.4 Animals
- Wistar albino rats of either sex were obtained from the animal house of the department. They were housed in an environmentally regulated room on a 12 hours light:12 hours dark cycle at 25 ± 2°C, and had free access to food and water. The experimental protocol was approved by the Institutional Animal Ethics Committee of the Institute and experiments were conducted according to the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India (CPCSEA-837/ac/2004) guidelines on the use and care of experimental animals.
- 2.5 Acute toxicity study
- Different doses [5 mg/kg, 50 mg/kg, 300 mg/kg, and 2000 mg/kg, by mouth (p.o.)] of EDC were given to the animals and were used to study acute toxicity in accordance to Organization for Economic Cooperation Development [17] guideline 423. Three female rats, each sequentially dosed at intervals of 48 hours, were used for the test. Once daily cage side observations included changes in skin, fur, eyes, mucous membrane (nasal), autonomic (salivation, lacrimation, perspiration, piloerection, urinary incontinence, and defecation), and central nervous system (drowsiness, gait, tremors, and convulsions) changes. Mortality, if any, was determined over a period of 2 weeks.
- 2.6 Selection of doses
- For the assessment of activity, two dose levels were chosen in such a way that the high dose was approximately one-tenth of the maximum dose during acute toxicity studies, and a low dose, which was 50% of the one-tenth dose (100 mg/kg, 200 mg/kg, p.o.).
- 2.7 Pharmacological assessment
- Assessment of EDC as an antisecretory and in-vivo antacid was carried out with a pyloric ligation induced ulcer model. The gastroprotective effect was assessed with an absolute ethanol induced ulcer model. Integrity of the gastric mucosa was evaluated with the estimation of glutathione (GSH) and gastric mucus level and using histopathological examination of gastric mucosal cells. The in-vitro antacid capacity was evaluated using a titration method. The effect of the extract on the liver was assessed by measuring serum biochemical parameters.
- 2.8 Pyloric ligation induced ulcers
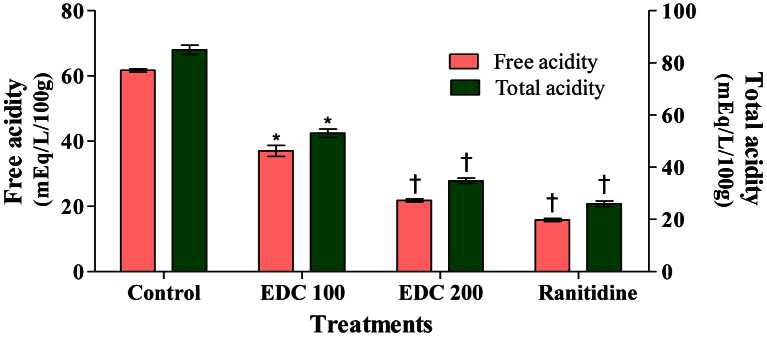
- EDC in doses of 100 mg/kg and 200 mg/kg and ranitidine of 50 mg/kg were administered orally for 7 days in their respective groups. The control group of animals received a suspension of 1% w/v carboxy methyl cellulose in distilled water and rats were kept fasting for 18 hours. Pyloric ligation was carried out in anesthetized rats to induce gastric ulceration [18]. The last dose of drugs was administered orally 1 hour before pyloric ligation, and 4 hours after pyloric ligation the rats were sacrificed. The stomach was dissected out and the gastric juice was drained into a small beaker and the stomach was opened along the greater curvature. The ulcer index was determined using the following scoring system: 0 = normal mucosa; 0.5 = blushing; 1 = spot ulcers; 1.5 = hemorrhage streaks; 2 = 3 mm < ulcers <5 mm; and 2.5 = ulcers >5 mm [19]. The gastric juice was centrifuged for 10 minutes at 2000 rpm. The supernatant was collected and used for the estimation of the volume of gastric juice, pH, free acidity, and total acidity. The volume was noted and expressed as mL/100 g/4 hours and the pH was measured using a pH meter. Estimation of free and total acidity of gastric juice was carried out as described by Card and Marks [20]. Free acidity and total acidity were determined by titrating with 0.01N sodium hydroxide using Topfer's reagent and phenolphthalein as an indicator respectively. The acidity was expressed as mEq/L/100g and acid output as mEq/100 g/4 hours 21, 22.
- 2.9 Absolute ethanol induced ulcers
- The experiment was performed according to the method of De-Andrade et al [23] with some modification. The food and water given to rats were withdrawn for 36 hours and 12 hours, respectively, before the commencement of the experiment. These rats were randomly divided into four equal groups (n = 6/group) and treated orally in the following manner: each rat in group 1 received 1 mL of 1% carboxy methyl cellulose solution. Animals in groups 2, 3, and 4 were administered with EDC 100 mg/kg, 200 mg/kg and ranitidine 50 mg/kg p.o., respectively. One hour after treatment, all the rats received 1 mL of absolute ethanol to induce a gastric ulcer. One hour later, the animals were sacrificed with a cervical dislocation, and all stomachs were removed and opened along the greater curvature. Each stomach was gently rinsed with water to remove the gastric contents and ulcers were graded as mentioned earlier.
- 2.10 Estimation of nonprotein sulfhydryl content in stomach tissues
- All groups of rats treated were utilized to estimate the GSH content in stomach tissues as nonprotein sulfhydryls according to the method described by Sedlak and Lindsay [24]. Glandular segment from each stomach was homogenized in 5 mL ice-cold 0.02M ethylenediaminetetraacetic acid solution. Aliquots (4 mL) of tissue homogenate were mixed with 3.2 mL of distilled water and 0.8 mL of 50% (w/v) trichloroacetic acid (50%) in glass tubes and centrifuged at 3000 rpm for 15 minutes, 2 mL supernatant was mixed with 4 mL Tris buffer (0.4M, pH 8.9) and 5,5’-dithio-bis(2-nitrobenzoic acid) (0.01M) was added. After shaking the reaction mixture, the absorbance was measured at 412 nm within 5 minutes of the addition of 5,5’-dithio-bis(2-nitrobenzoic acid) against a blank with no homogenate.
- 2.11 Estimation of gastric wall mucus
- Gastric wall mucus was determined according to the method of Corne et al [25]. The glandular segment from the stomach was removed, weighed, and incubated in tubes containing 1% Alcian blue solution (0.16M sucrose in 0.05M sodium acetate, pH 5.8) for 2 hours. The Alcian blue binding extract was centrifuged and the absorbance of supernatant was measured at 498 nm. The quantity of Alcian blue extracted (μg/g of glandular tissue) was then calculated.
- 2.12 Effects on the liver
- The functioning of the liver was assayed by evaluating the alanine transaminase, aspartate transaminase, alkaline phosphatase, total protein, albumin, bilirubin direct and bilirubin total.
- 2.13 Histopathology
- The samples of the stomach from different groups were preserved in 10% buffered formalin and processed for routine paraffin block preparation. Sections of thickness of approximately 5 μm were cut and stained with hematoxylin and eosin. These were examined under the microscope for histopathological changes such as degeneration, hemorrhage, edematous appearance, erosion and necrosis.
- 2.14 Antacid capacity
- In the in-vitro model, the acid neutralizing capacity of an antacid is the amount of hydrochloric acid that it can neutralize. Thirty-milliliters of 1.0N hydrochloric acid volumetric standard (VS) were added into the test preparation (i.e., EDC) with continuous stirring with a magnetic stirrer for 15 minutes. After this, the titration is started immediately (period should not be exceeded to additional 5 minutes) and the excess hydrochloric acid is titrated with 0.5N sodium hydroxide VS to attain a stable pH (for 10–15 seconds) of 3.5. The number of mEq of acid consumed is calculated with the formula: total mEq = (30 × NHCl) – (VNaOH × NNaOH) in which NHCl and NNaOH are the normalities of the hydrochloric acid VS and the sodium hydroxide VS, respectively; and VNaOH is the volume of sodium hydroxide VS used for titration. The result is expressed in terms of mEq of acid consumed per gram of the substance tested [26].
- 2.15 Statistical analysis
- The results were expressed as mean ± standard error of the mean and were analyzed using one-way analysis of variance followed by Dunnett's test using GraphPad Prism 5.0 (Graph-Pad Software Inc., San Diego, California, USA). A value of p < 0.05 was considered statistically significant.
Materials and methods
- 3.1 Preliminary phytochemical screening
- The qualitative phytochemical result shows the presence of carbohydrates, proteins, glycosides, phenolics, tannins, alkaloids and flavonoids.
- 3.2 Acute toxicity study
- The EDC was found to be safe up to 2000 mg/kg with no signs of mortality or change in behavioral pattern. This result suggests that plant extract is not toxic and is safe.
- 3.3 Pyloric ligation induced ulcer
- Gastric secretion was evaluated as gastric juice volume, pH, free acidity, and total acidity for 4 hours after pyloric ligation. Rats were pretreated and the last dose was administered immediately 1 hour before ligation. EDC extract (100 mg/kg and 200 mg/kg) and ranitidine decreased the gastric juice volume, free acidity, and total acidity, and increased the pH. This decrease reached statistical significance at doses of EDC 200 mg/kg and ranitidine for the volume, free acidity, and total acidity for 4 hours after pyloric ligation (Figures 1 and 2). Gastric mucus content level did not show significant changes in response to any of the tested substances (Figure 3). Neither EDC nor ranitidine modified the GSH level for 4 hours after pylorus ligation to the rats (Figure 4). The ulcer index reduces to 3.25 ± 0.51 in EDC 200 mg/kg and 3.08 ± 0.51 in ranitidine treated groups (p < 0.001) and is shown in Table 1.
- 3.4 Absolute ethanol induced ulcer
- Oral administration of absolute ethanol produced severe hemorrhagic lesions in glandular mucosa. The control rats had an ulcer index of 17.00 ± 1.06. In animals pretreated with EDC at doses of 100 mg/kg and 200 mg/kg, a significant inhibition of ethanol mucosal injury was detected, showing an ulcer index of 6.83 ± 0.61 and 5.17 ± 0.70, respectively. The ranitidine showed an inhibition of lesion formation (71.57%; Table 2). The gastric wall mucus and GSH level were increased in EDC 200 mg/kg and ranitidine treated rats (Figure 3, Figure 4).
- 3.5 Effect of extract on liver
- Blood serum levels of alanine transaminase (p < 0.01) were increased significantly in rats in both types of ulcer model, were replenished by EDC in both doses, and were statistically significant (p < 0.05). Other parameters, aspartate transaminase, alkaline phosphatase, total protein, albumin, bilirubin direct and bilirubin total were in the normal range. The data are presented in Table 3.
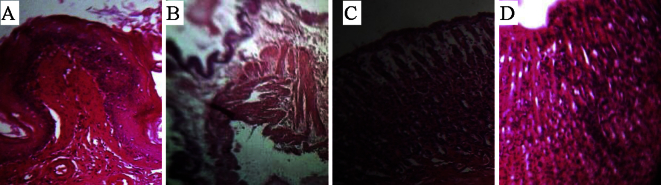
- 3.6 Histopathology study
- Histopathological studies (Figure 5A–5D and Figure 6A–6D) further confirmed that pretreatment with EDC at 200 mg/kg inhibited pyloric ligated and absolute ethanol ulcer, congestion, edema, hemorrhage and necrosis in gastric mucosa. In reducing congestion and hemorrhage, the EDC (200 mg/kg, p.o.) efficacy was comparable to that of ranitidine.
- 3.7 Antacid capacity of EDC
- Twenty-milliequivalents and 26 mEq of acid were consumed per gram of ethanol extract from roots of D. carota and gelusil, respectively.
Results
- Anatomical and functional integrity of the gastric mucosa is dependent on the balance between aggressive and defensive factors. The accomplishment of pharmacological treatments in preventing or healing ulcers may not depend only on the inhibition of acid secretion, but also on the enhancement of mucosal protective factors [1].
- The present study shows that the aqueous ethanol extract from D. carota possesses gastroprotective activity as evidenced by its significant inhibition of the development of ulcers induced by physical and chemical agents. Pyloric ligated induced ulcer is thought to be due to the increased presence of acid in the stomach. The ligation of the pyloric end of the stomach causes accumulation of gastric acid in the stomach, thus, agents that decrease the gastric acid secretion and/or increase mucus secretion are effective in preventing the ulcers induced by this method [27]. In this method, several parameters such as gastric wall mucus content, and gastric contents pH, volume, and hydrogen ion concentration were evaluated in animals after pretreatment with EDC at various doses. EDC exhibits antiulcerogenic activity by significantly reducing the pH, volume and total acidity, and without altering the gastric wall mucus much compared with the control group.
- The occurrence of ethanol induced ulcers, which is predominant in the glandular part of the stomach, was previously reported to stimulate the formation of reactive oxygen species, resulting in damage to rat gastric mucosa [28]. EDC has prevented ethanol induced exhaustion of gastric wall mucus. Mucus, in conjunction with bicarbonate secreted by surface epithelial cells, has long been thought to serve a key role in shielding the gastric epithelium from damage induced by acid and pepsin [29]. In addition, mucus plays a vital role in preventing bacterial colonization and translocation to the luminal surface; furthermore, it plays a key role in the deterrence of mechanical damage to the epithelium, providing a microenvironment over sites of superficial injury in which rapid repair can occur [30]. Also, in this model, oxygen derived reactive species are associated with gastrointestinal damage, and antioxidants prevent the lesions by various agents. Ethanol appears to deplete the level of nonprotein sulfhydryl content, such as GSH, in stomach tissues. GSH is a tripeptide, which acts as an antioxidant and seems to be important for the maintenance of the mucosal integrity in the stomach by scavenging reactive oxygen species either directly or enzymatically via glutathione peroxidase. Therefore, depletion of gastric mucosal GSH may result in the accumulation of free radicals that can initiate membrane damage [31].
- In conclusion, this study showed potent antisecretory and gastroprotective activities of Daucus carota in pyloric ligation, absolute ethanol induced gastric ulcers. This indicates its therapeutic potential to be used as a cost effective herbal antiulcer agent. The precise mechanisms of action involving these protective factors need to be studied.
Discussion
- We declare that we have no conflict of interest.
Conflicts of interest
-
Acknowledgements
- The authors wish to thank Professor (Dr) R.M. Dubey, Vice Chancellor, IFTM University, Moradabad, for his generous support of the study. This study is part of a PhD from UP Technical University, Lucknow.
Acknowledgments
-
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Article information
- 1. Lapa F.R., Freitas C.S., Baggio C.H.. Gastroprotective activity of the hydroalcoholic extract obtained from Polygala paniculata L. in rats. J Pharm Pharmacol 59(10). 2007 Oct;1413−1419. PMID: 17910817.ArticlePubMed
- 2. Hoogerwerf W.A., Pasricha P.J.. Edited by Brunton L.L., Lazo J.S., Parker K.L.: The pharmacological basis of therapeutics. 2006. Mc Graw Hill; New York (NY): pp 967−981.
- 3. Zakaria Z.A., Hisam E.E.A., Rofiee M.S.. In vivo antiulcer activity of the aqueous extract of Bauhinia purpurea leaf. J Ethnopharmacol 137(2). 2011 Sep;1047−1054. PMID: 21802502.ArticlePubMed
- 4. Nadkarni K.M.. Indian Materia Medica. Vol. I:1976. Popular Prokashan; Bombay: p 442.
- 5. Kirtikar K.R., Basu B.D.. Indian medicinal plants. Vol. II:2006. International Book Distributors; Dehradun (Uttanchal): pp 1229−1231.
- 6. Prochezhian E., Ansari S.H.. Analgesic and anti-inflammatory activity of volatile oil from Daucus carota Linn. Indian J Nat Prod 16(1). 2000;24−26.
- 7. Prakash A.O.. Biological evaluation of some medicinal plant extracts for contraceptive efficacy in females. Contracept Fertil Sex 13(4). 1984 Apr;649−655.Article
- 8. Abou Zeinab R.M., Mroueh M., Daher C.F.. Potent anti-tumor promoting effects of Daucus carota oil extract in mice. Planta Med 74:2008;1008. Article
- 9. Bishayee A., Sarkar A., Chaterjee M.. Hepatoprotective activity of carrot against carbon tetrachloride intoxication in mouse liver. J Ethnopharmacol 47(2). 1995 Jul;69−74. PMID: 7500638.ArticlePubMed
- 10. Neef H., Declercq P., Laekeman G.. Hypoglycemic activity of selected European plants. Phytother Res 9(1). 1995 Feb;45−48.Article
- 11. Patil M.V.K., Kandhare A.D., Bhise S.D.. Anti-inflammatory effect of Daucus carota root on experimental colitis in rats. Int J Pharm Pharm Sci 4(1). 2012;337−343.
- 12. Wehbe K., Mroueh M., Daher C.F.. The potential role of Daucus carota aqueous and methanolic extracts on inflammation and gastric ulcers in rats. J Complement Integr Med 6(1). 2009 Mar;7. Article
- 13. Chopra R.N., Chopra I.C., Verma B.S.. Supplement to glossary of Indian medicinal plants. 2005. National Institute of Science Communication and Information Resources; New Delhi (India): p 23.
- 14. The Wealth of India . Raw Materials Vol.III: D–E. 2003. National Institute of Science and Information Resources, CSIR; New Delhi (India): pp 19−23.
- 15. Trease G.E., Evans W.C.. A text book of pharmacognosy. 1987. ELSB Baillere Tindal; Oxford (UK): p 1055.
- 16. Khandelwal K.R.. Practical pharmacognosy. 21st rev. 2011. Nirali Prakashan; Pune (India): pp 25.1−25.9.
- 17. OECD . Acute oral toxicity–acute oral toxic class method, guideline for the testing of chemicals. OECD 423; Adopted: 17 December 2001. 2001. OECD; Paris (France): pp 1−14.
- 18. Shay H., Komarov S.A., Fels S.S.. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterol 4:1945;43−61.
- 19. Kulkarni S.K.. Handbook of experimental pharmacology. 3rd ed.1999. Vallabh Prakashan; Delhi (India): pp 148−150.
- 20. Card W.I., Marks I.N.. The relationship between the acid output of the stomach following “maximal” histamine stimulation and the parietal cell mass. Clin Sci 19:1960 Feb;147−163. PMID: 13807647.PubMed
- 21. Chandra P., Sachan N., Kishore K.. Acute, sub-chronic oral toxicity studies and evaluation of antiulcer activity of Sooktyn in experimental animals. J Adv Pharm Tech Res 3(2). 2012 Apr;117−123.Article
- 22. Devi R.S., Narayan S., Vani G., Devi C.S.S.. Gastroprotective effect of Terminalia arjuna bark on diclofenac sodium induced gastric ulcer. Chem Biol Interact 167(1). 2007 Apr;71−83. PMID: 17327128.ArticlePubMed
- 23. De-Andrade S.F., Lemos M., Comunello E.. Evaluation of the antiulcerogenic activity of Maytenus robusta (Celastraceae) in different experimental ulcer models. J Ethnopharmacol 113(2). 2007 Sep;252−257. PMID: 17629427.ArticlePubMed
- 24. Sedlak J., Lindsay R.H.. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem 25(1). 1968 Oct;192−205. PMID: 4973948.ArticlePubMed
- 25. Corne S.J., Morrissey S.M., Woods R.J.. A method for the quantitative estimation of gastric barrier mucus. J Physiol 242(2). 1974 Oct;116−117.
- 26. USP: United States Pharmacopeia-National Formulary XV. 2007. US Pharmacopoeial Convention; Rockville (MD).
- 27. Khare S., Asad M., Dhamanigi S.S.. Antiulcer activity of cod liver oil in rats. Indian J Pharmacol 40(5). 2008 Oct;209−214. PMID: 20040959.ArticlePubMed
- 28. Amresh G., Zeashan H., Gupta R.J.. Gastroprotective effects of ethanolic extract from Cissampelos pareira in experimental animals. J Nat Med 61(3). 2007 Jul;323−328.Article
- 29. Tortora G.J., Derrickson B.. Principles of anatomy and physiology. 12th ed.2009. John Wiley & Sons Inc; Somerset (USA): pp 937−942.
- 30. Wallace J.L.. Mechanisms of protection and healing: current knowledge and future research. Am J Med 110(1). 2001 Jan;S19−23.Article
- 31. Silva M.I.G., Moura B.A., Aquino M.R.. Gastroprotective activity of isopulegol on experimentally induced gastric lesions in mice: investigation of possible mechanisms of action. Naunyn Schmied Arch Pharmacol 380(3). 2009 Sep;233−245.Article
References




| Treatment | Dose (mg/kg) | Ulcer index (mean ± SEM)∗ | % inhibition |
|---|---|---|---|
| Control | 1 mL | 14.33 ± 1.20 | – |
| EDC | 100 | 5.92 ± 0.45† | 58.71 |
| EDC | 200 | 3.25 ± 0.51† | 77.32 |
| Ranitidine | 50 | 3.08 ± 0.51† | 78.48 |
| Treatment | Dose (mg/kg) | Ulcer index (mean ± SEM)∗ | % inhibition |
|---|---|---|---|
| Control | 1 mL | 17.00 ± 1.06 | – |
| EDC | 100 | 6.83 ± 0.61† | 59.80 |
| EDC | 200 | 5.17 ± 0.70‡ | 69.61 |
| Ranitidine | 50 | 4.83 ± 0.86‡ | 71.57 |
| Treatment | Pyloric ligation ulcer∗ | Alcohol-induced ulcer∗ |
|---|---|---|
| Control | 31.42 ± 2.23 | 32.23 ± 2.32 |
| EDC (100 mg/kg) | 23.24 ± 1.64† | 24.12 ± 4.26† |
| EDC (200 mg/kg) | 22.64 ± 2.15† | 23.72 ± 2.52† |
Figure & Data
References
Citations

- Plants and their Bioactive Compounds as a Possible Treatment for Traumatic Brain Injury-Induced Multi-Organ Dysfunction Syndrome
Manisha Thakur, Neeru Vasudeva, Sunil Sharma, Ashok Kumar Datusalia
CNS & Neurological Disorders - Drug Targets.2023; 22(9): 1313. CrossRef - Gastroprotective evaluation of Medicago sativa L. (Fabaceae) on diabetic rats
Phool Chandra, Mohammad Kaleem, Neetu Sachan, Rashmi Pathak, Ashwag S. Alanazi, Nawaf A. Alsaif, Sary Alsanea, Bader Alsuwayt, Mohammed M. Alanazi, Atul Kabra
Saudi Pharmaceutical Journal.2023; 31(11): 101815. CrossRef - ВИВЧЕННЯ ХІМІЧНОГО СКЛАДУ ТА БІОЛОГІЧНОЇ АКТИВНОСТІ МОРКВИ ПОСІВНОЇ (DAUCUS CAROTA L. VAR. SATIVUS). ОГЛЯД ЛІТЕРАТУРИ
Л. П. Морозова
ПРОДОВОЛЬЧІ РЕСУРСИ.2023; 11(20): 72. CrossRef - Isolation of Thymol from Trachyspermum ammi Fruits for Treatment of Diabetes and Diabetic Neuropathy in STZ-Induced Rats
Neetu Sachan, Nikita Saraswat, Phool Chandra, Mohammad Khalid, Atul Kabra, Riaz Ullah
BioMed Research International.2022; 2022: 1. CrossRef - Antioxidant and Gastroprotective Activity of Suaeda fruticosa Forssk. Ex J.F.Gmel
Afsheen Ayaz, QurratUlAin Jamil, Musaddique Hussain, Fayyaz Anjum, Adeel Sarfraz, Taha Alqahtani, Nadia Hussain, Reem M. Gahtani, Ayed A. Dera, Hanan M. Alharbi, Shahid M. Iqbal
Molecules.2022; 27(14): 4368. CrossRef - Hydromethanolic Crude Extract of the Leaf of Urtica simensis Hochst. ex. A. Rich. (Urticaceae) Acquires Appreciable Antiulcer Effect: Validation for In Vivo Antiulcer Activity
Woretaw Sisay, Yared Andargie, Mulugeta Molla, Alefe Norahun, Mozaniel Oliveira
Evidence-Based Complementary and Alternative Medic.2021; 2021: 1. CrossRef - The influence of a simulated digest of an equine dietary feed additive G's formula on contractile activity of gastric smooth muscle in vitro
Jennifer L. MacNicol, Coral Murrant, Wendy Pearson
Journal of Animal Physiology and Animal Nutrition.2020; 104(6): 1919. CrossRef Evaluation of the Anti-Ulcer Activity of Hydromethanolic Crude Extract and Solvent Fractions of the Root of Rumex nepalensis in Rats
Woretaw Sisay Zewdu, Tezera Jemere Aragaw
Journal of Experimental Pharmacology.2020; Volume 12: 325. CrossRef- Standardization Using Analytical Techniques (UV, NMR, FTIR, HPLC, Mass) and Pharmacognostic Evaluation of the Roots of Selinum vaginatum: A Rare Himalayan Plant of the Rohtang Region
Nikita Saraswat, Neetu Sachan, Phool Chandra
Current Biotechnology .2020; 9(2): 89. CrossRef - Cytoprotective, antioxidant and anti-inflammatory mechanism related to antiulcer activity of Cissampelos sympodialis Eichl. in animal models
Igor Rafael Praxedes De Sales, Rodrigo De Oliveira Formiga, Flávia Danniele Frota Machado, Raphaela Francelino Nascimento, Matheus Marley Bezerra Pessoa, Monique Emanuela Frutuoso Xavier Barros, Giciane Carvalho Vieira, Francisco Allysson Assis Ferreira G
Journal of Ethnopharmacology.2018; 222: 190. CrossRef - Antiulcer properties of fruits and vegetables: A mechanism based perspective
Choudhary Harsha, Kishore Banik, Devivasha Bordoloi, Ajaikumar B. Kunnumakkara
Food and Chemical Toxicology.2017; 108: 104. CrossRef - Glutathione sulfotransferase inhibition activity of a self-fermented beverage,Kanji
Abida Latif, Khalid Hussain, Naureen Shehzadi, Muhammad Islam, Muhammad Tanveer Khan, Rukhsana Anwar, Humaira Majeed Khan, Nadeem Irfan Bukhari
Pharmaceutical Biology.2017; 55(1): 547. CrossRef - Mild Hypothermia Protects Pigs’ Gastric Mucosa After Cardiopulmonary Resuscitation via Inhibiting Interleukin 6 (IL-6) Production
Yan Wang, Jian Song, Yuhong Liu, Yaqiang Li, Zhengxin Liu
Medical Science Monitor.2016; 22: 3523. CrossRef


 PubReader
PubReader Cite
Cite
