Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(4); 2023 > Article
-
Short Communication
Trends of legionellosis reported in Jeju Province, Republic of Korea, 2015–2022 -
Juyoung Park1,2
 , Jong-Myon Bae2,3
, Jong-Myon Bae2,3
-
Osong Public Health and Research Perspectives 2023;14(4):321-327.
DOI: https://doi.org/10.24171/j.phrp.2023.0145
Published online: August 21, 2023
1Department of Medicine, Graduate School, Jeju National University, Jeju, Republic of Korea
2Jeju Center for Infectious Disease Control and Prevention, Jeju, Republic of Korea
3Department of Preventive Medicine, Jeju National University College of Medicine, Jeju, Republic of Korea
- Corresponding author: Jong-Myon Bae Department of Preventive Medicine, Jeju National University College of Medicine, 102 Jejudaehak-ro, Jeju 63243, Republic of Korea E-mail: jmbae@jejunu.ac.kr
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 1,201 Views
- 98 Download
Abstract
-
Objectives
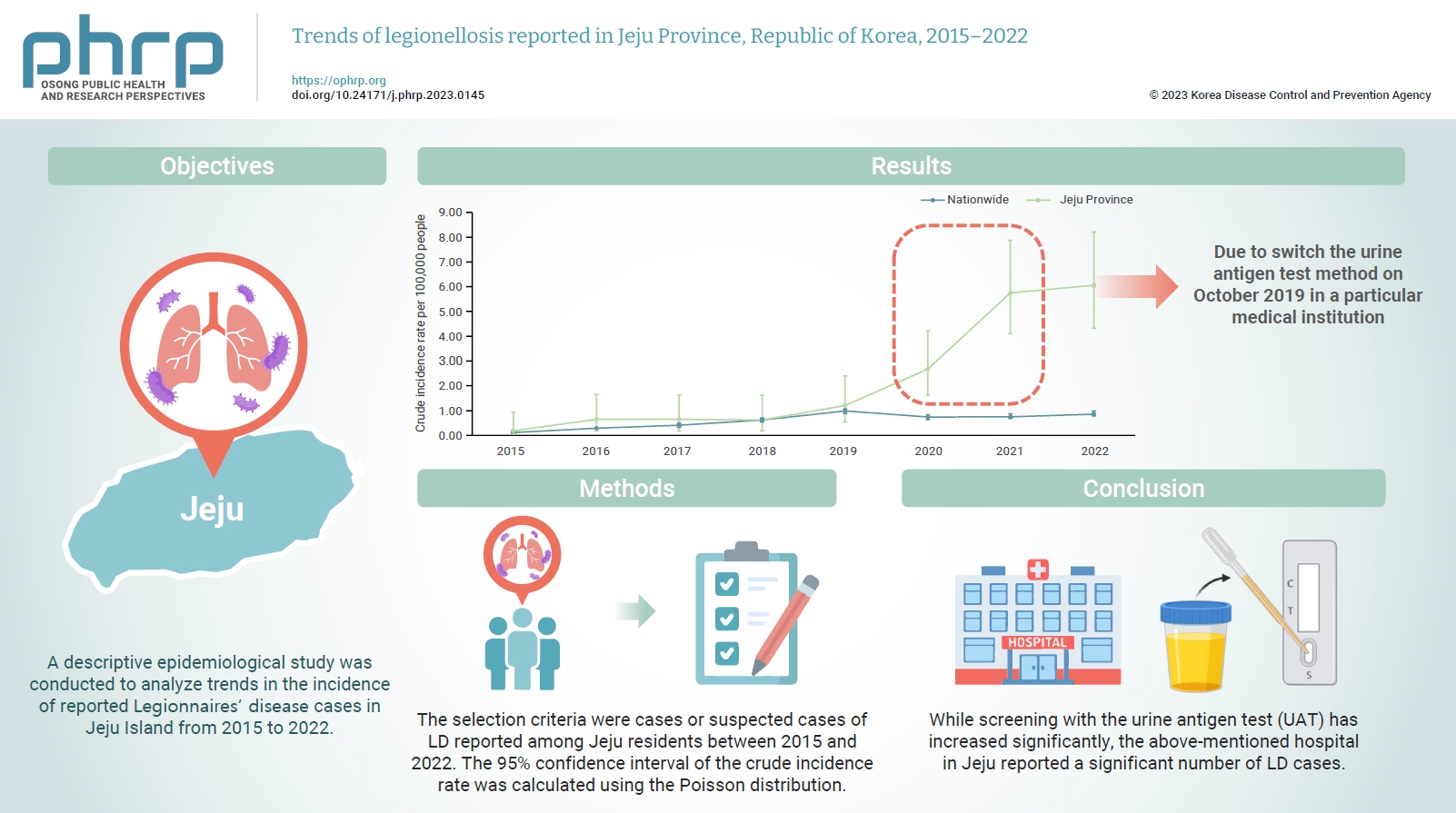
- The number of reported cases of Legionnaires’ disease (LD) in the Republic of Korea surged nationally in 2016; however, in 2022, this number was higher in Jeju Province than the previous national peak. A descriptive epidemiological study was conducted to analyze trends in the incidence of reported LD cases in Jeju Island from 2015 to 2022.
-
Methods
- The data for this study were obtained from case reports submitted to the Korea Disease Control and Prevention Agency through its Disease and Health Integrated Management System. The selection criteria were cases or suspected cases of LD reported among Jeju residents between 2015 and 2022. The 95% confidence interval of the crude incidence rate was calculated using the Poisson distribution.
-
Results
- Since 2020, the incidence rate of LD in Jeju has risen sharply, showing a statistically significant difference from the national incidence rate. A particular medical institution in Jeju reported a significant number of LD cases. Screening with the urine antigen test (UAT) also increased significantly.
-
Conclusion
- Our findings indicate that the rapid increase in cases of LD in Jeju Province since 2020 was due to the characteristics of medical-care use among Jeju residents, which were focused on a specific medical institution. According to their clinical practice guidelines, this medical institution conducted UATs to screen patients suspected of pneumonia.
- The leading causative agent of Legionnaires’ disease (LD) is Legionella pneumophilia serotype 1, which accounts for 2% to 15% of cases of community-acquired pneumonia [1−4]. LD presents as an atypical type of pneumonia because the patient often has no fever and can have extrapulmonary clinical symptoms, such as headache or diarrhea [5−7].
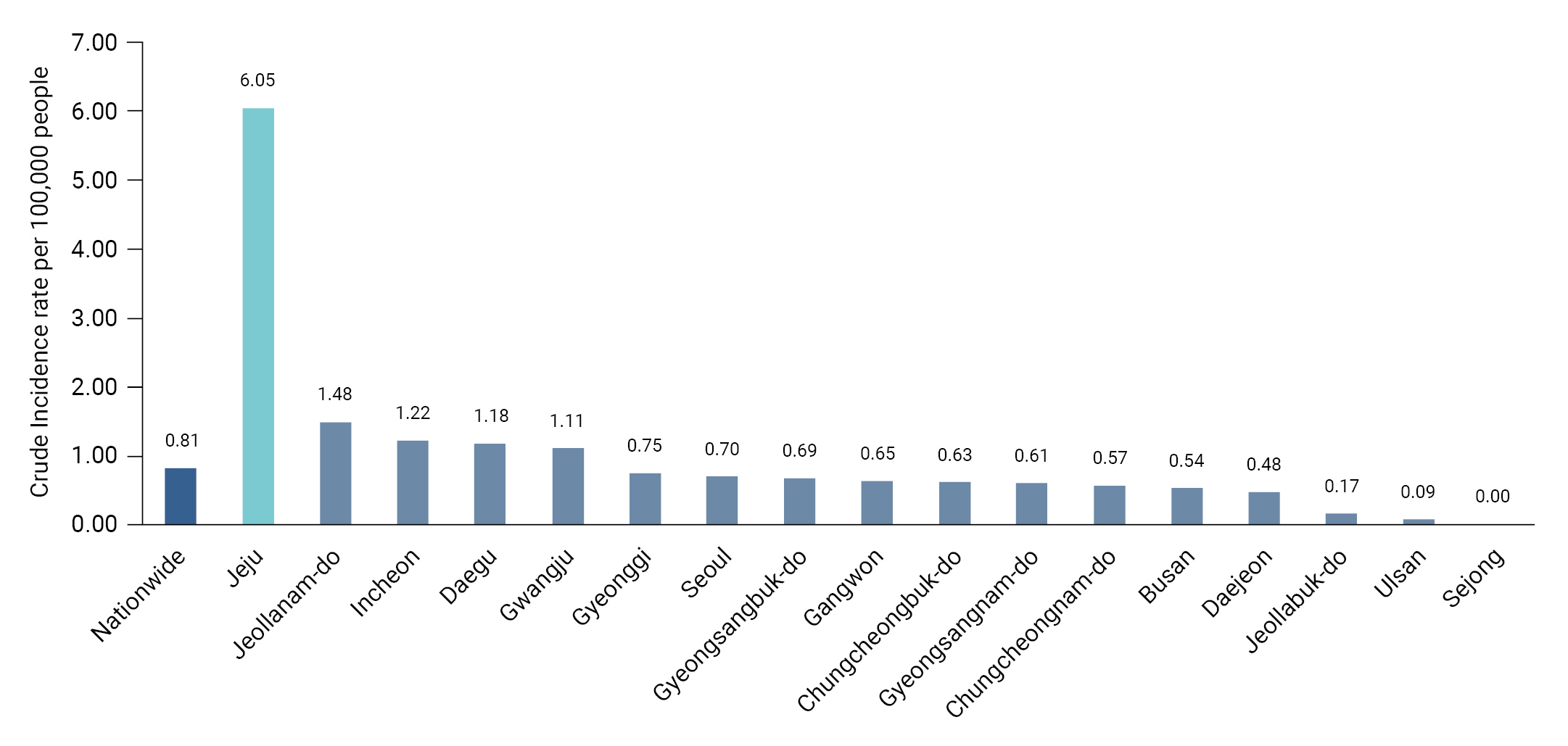
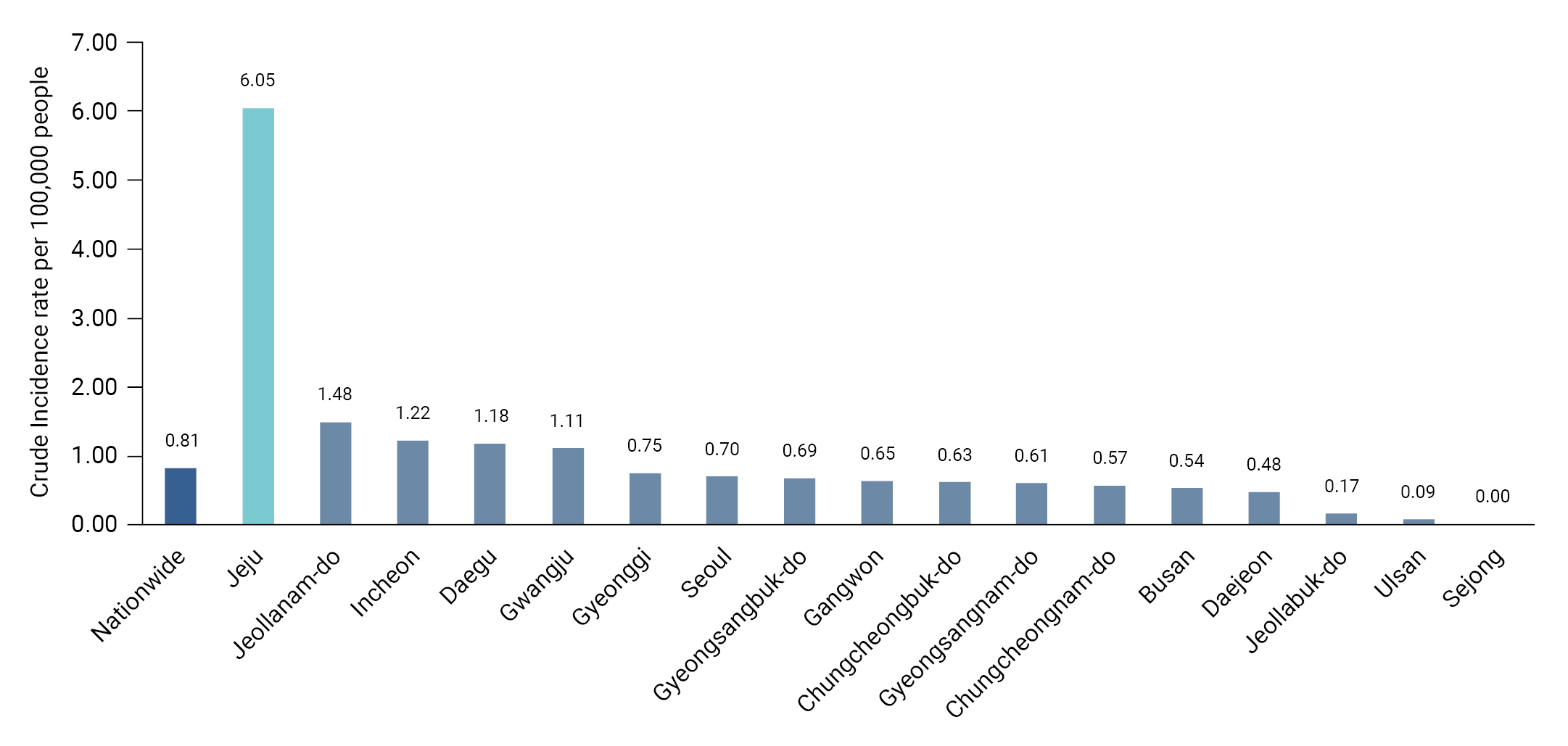
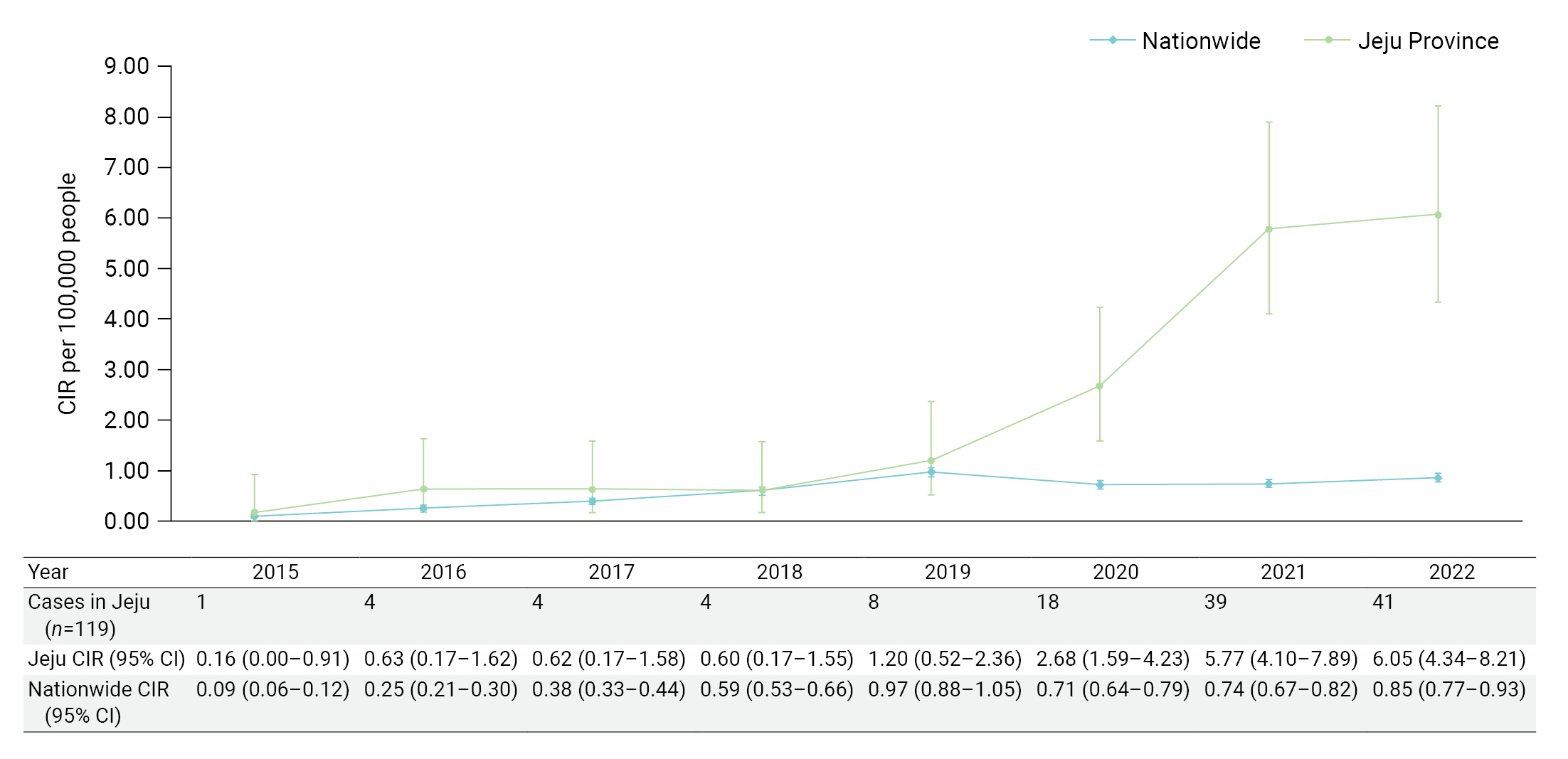
- Although the incidence rate of LD varies widely among countries [8], it has increased significantly worldwide, including in the United States, Europe, and Asia, since 2000 [9−11]. After LD was designated a reportable infectious disease in the Republic of Korea in 2000, the number of reported cases increased from 45 in 2015 to 128 in 2016 [12]. The national reporting rates for the corresponding years were 0.09 and 0.25 per 100,000 people, respectively, representing a 2.7-fold increase over a 1-year period. The 2022 reporting rate in Jeju Province was 6.05 per 100,000 people, the highest rate among local governments and 7.5 times higher than the national rate (0.81 per 100,000) (Figure 1) [13].
- While there was a sudden increase in reports of LD nationwide in 2016, an explanation was needed for the surge in reported LD cases in Jeju Province in 2022, as compared to the rest of the nation. Therefore, we conducted a descriptive epidemiological study on trends in the LD reporting rate in Jeju Province from 2015 to 2022.
Introduction
- The data for this study were sourced from legionellosis case reports submitted through the Disease and Health Integrated Management System (https://is.kdca.go.kr) of the Korea Disease Control and Prevention Agency (KDCA). For this epidemiological investigation, residents of Jeju Province were selected as study subjects among the cases classified as LD patients or suspected LD patients from 2015 to 2022.
- For cases that met the selection criteria, sex, age, occurrence date, reporting institution, test method for LD diagnosis, and route of infection were extracted. Subjects were classified into 2 age groups (<65 years and ≥65 years) based on their age at the time of reporting. The date of LD occurrence was defined as the date of sample collection, and if the date of sample collection was not reported, it was based on the date of reporting at the medical institution. The infection route was divided into nosocomial and non-nosocomial infections based on descriptions in the case report. If the route of infection was unknown, patients in whom suspicious symptoms began at least 2 days after the hospitalization date were considered to have nosocomial infections. Infections acquired in the community or at home without a history of hospitalization were considered non-nosocomial.
- To calculate the annual crude incidence rate (CIR) of LD, we used the annual mid-year population based on the resident registration used by the KDCA [13]. The 95% confidence interval (CI) of the CIR was calculated by applying the Poisson distribution. The 2-sample t-test, chi-square test, and Fisher exact test were performed to evaluate differences in the characteristics of related variables between the classified groups. STATA ver. 17.0 (Stata Corp.) was used for the analysis, and p-values <0.05 indicated statistical significance.
- This study was approved for exemption by the institutional review board (IRB) of Jeju National University as a study utilizing secondary data (JJNU-IRB-2023-019).
Materials and Methods
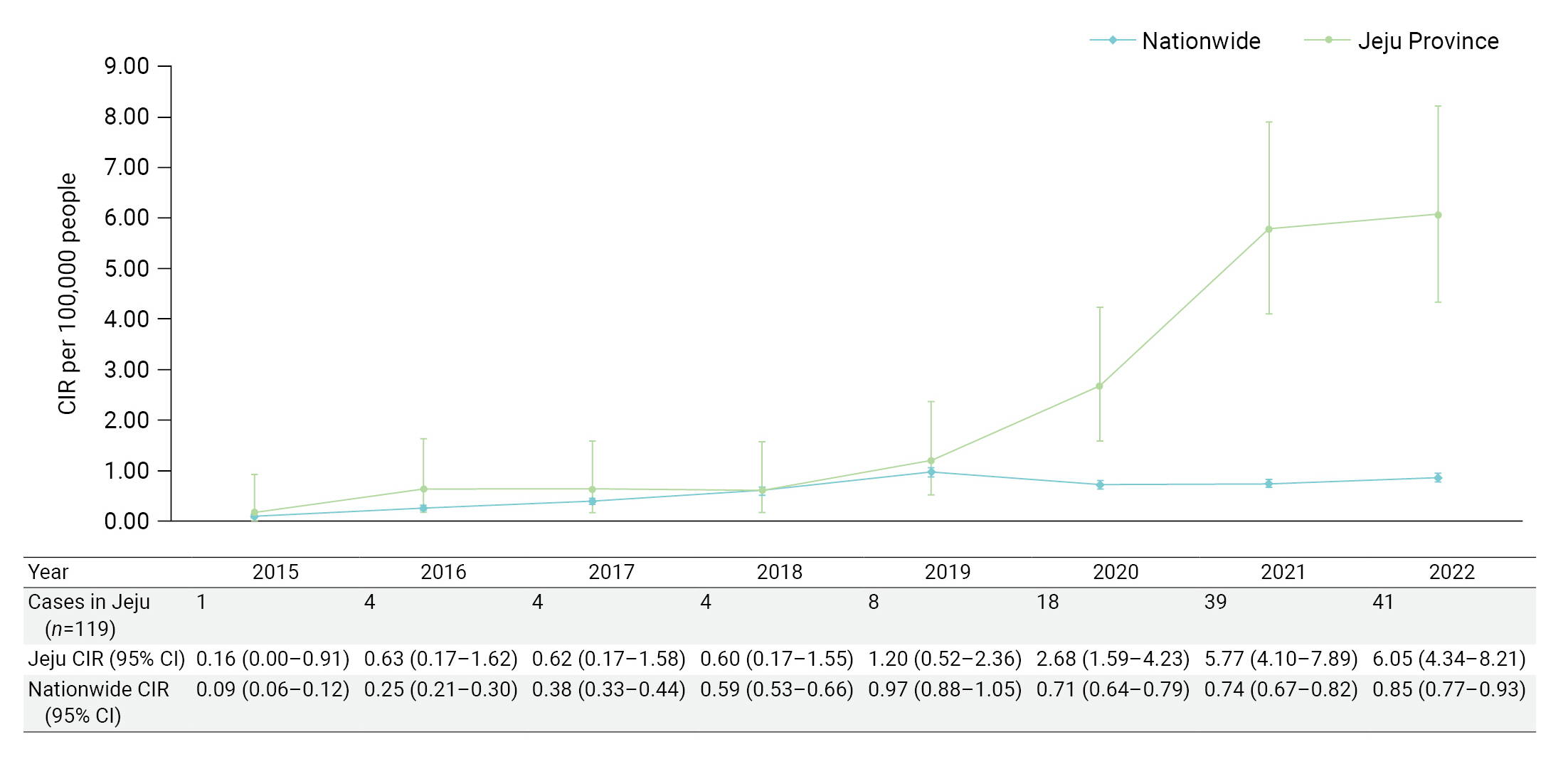
- We analyzed 119 patients who met the selection criteria. The distribution of occurrences and the CIR by year is shown in Figure 2. Since 2020, the incidence rate of LD in Jeju Province has risen sharply, showing a statistically significant difference from the national incidence rate. In 2022, the LD incidence rate was the highest in Jeju Province and was 7.5-fold higher than the national incidence rate (6.05/0.81). Although there was no significant difference between the sexes in the distribution of incidence rates by year, the incidence rate in the older group (≥65 years) was significantly higher after 2020 (Table 1).
- The epidemiological characteristics of reported cases before and after a sharp change in the incidence rate (before 2019 and after 2020) were compared (Table 2). With the increased number of cases (from 21 to 98) the mean age at occurrence differed significantly (63.8 vs. 76.8 years) (p<0.001 by two-sample t-test). There was no significant difference between the sexes or routes of infection. One medical institution reported a significantly higher number of LD cases than other institutions (p<0.05 by the chi-square test), and the urine antigen test (UAT) was used significantly more frequently as the test method at that institution (p<0.05 by the Fisher exact test).
Results
- In this study, we found that the patient age in cases of LD in Jeju Province increased from 2020 onwards, while the distribution of sex and route of infection did not change significantly. We also found that intensive case reporting arose from a specific medical institution where application of the UAT had also increased.
- If the occurrence of a particular disease increases in a community, possible changes in reporting guidelines, increases in disease awareness, and the introduction of a new test method should be considered before deciding whether the incidence of the disease per se has increased [10,14,15]. First, in our context, the possibility of an increase due to changes in reporting guidelines could be preferentially excluded, as the diagnostic criteria for reporting in the KDCA’s Legionella management guidelines for 2012, 2016, 2018, 2019, and 2020 had not changed [12]. Second, in terms of increased awareness of LD, it is plausible that both the public and healthcare professionals had gained interest in pneumonia due to the sudden outbreak of coronavirus disease 2019 in 2020 [16]. However, since this change in interest was a nationwide phenomenon, it is not likely to explain the increased reporting of LD from Jeju Province as compared to the rest of the country. Last, the hypothesis that the increase in reported LD patients was due to the introduction of a new testing method is likely to be valid according to the simultaneous variability hypothesis-setting method [14], since the number of reports based on positive UAT results changed markedly around 2020.
- Since the development of a method for detecting urinary L. pneumophilia antigen in late 1990 [17], the UAT has become a major diagnostic method for LD worldwide [18] due to its easy handling and rapid diagnosis [19]. Amid these changes, the incidence rate of LD in the United States surged by 192% and 286% in 2009 and 2014, respectively, relative to the incidence rate in 2000 [15,20]. In Europe, the incidence rate was 2.6 times higher in 2015 than in 2000 [21,22]. In addition, the incidence rate in Hong Kong was 5.7 times higher in 2015 than in 2005. As discussed above, the predominant interpretation of these findings is that the rapid increase in the incidence rate of LD since 2000 reflects the introduction of the UAT [15,23−25]. This interpretation is notably supported by the fact that 97% of all cases in the United States were diagnosed by means of the UAT, while there was no change in the distribution of infection routes [15]. Therefore, it can be interpreted that the rapid increase in the reporting rate of nationwide LD since 2015 was also an effect of the introduction of the UAT. In our study, as seen in the United States [15], 87.4% of all cases were diagnosed via the UAT (104/119), while the distribution of infection routes in Jeju Province did not change (Table 2). Thus, the impact of the UAT introduction can explain the 37.8-fold (6.05/0.16) increase in the incidence rate from 2015 to 2022 in Jeju Province (Figure 2).
- It has also been argued that the number of LD patients increased rapidly after the introduction of the UAT, which led to diagnoses of more LD cases that had been missed previously [18]. In other words, due to the microbiological characteristics of LD pathogens, the incidence rate of LD before introducing the UAT method was underestimated [11,15,20]. LD is an atypical type of pneumonia, which often is not associated with fever and shows extrapulmonary clinical symptoms, such as headache or diarrhea, making it difficult to diagnose [6,7]. However, due to the high sensitivity of the UAT used in screening, the period from symptom onset to diagnosis was shortened by 5 days [26], and the mortality rate also decreased rapidly [24].
- If the UAT enabled the diagnosis of patients who would otherwise have been missed, it could explain why the reporting rate of Jeju Province was the highest among local governments in the Republic of Korea, for the following 3 reasons:
- First, a main finding of this study is that 88 of 98 LD cases reported in Jeju Province after 2020 were from a specific medical institution. In other words, that medical institution diagnosed and treated 90% of LD cases among Jeju residents.
- Second, in accordance with the Treatment Guidelines for Pneumonia in the Republic of Korea [27], the abovementioned medical institution had been performing the UAT for early diagnosis and initial antibiotic selection for all patients with suspected community-acquired pneumonia who required hospitalization. By actively performing UATs, with a sensitivity of 80% and specificity of 95% or higher [17], the diagnosis rate of LD, an atypical type of pneumonia, increased. This also supported a main finding of the present study—namely, that the age of confirmed LD patients had significantly increased since 2020. The reason for this finding is that individuals ≥65 years of age have more underlying diseases than those <65 years and have a relatively low clinical suspicion of pneumonia and a positive rate of general blood tests [28].
- Third, on October 7, 2019, the abovementioned hospital switched the UAT method from immunochromatographic assay-based UAT (ICT-UAT) to fluorescence immunoassay-based UAT (FIA-UAT). Since the examiner directly reads the results with the ICT-UAT method, subjective reading errors can result in false negatives. In contrast, in the FIA-UAT method, a scanner reads the results automatically [29] and can more sensitively diagnose faint bands that are difficult to distinguish with the eyes [19,30]. Hence, the fact that the hospital used the more sensitive FIA-UAT, which reduced false negative errors from the beginning of October 2019, supports the rapid increase in the number of LD cases since 2020.
- There were some limitations in this study. First, 10 cases that were finally classified as “non-LD” cases after further epidemiological investigation were excluded. Thus, the possibility of late exclusions should be considered among the subjects selected as LD cases. However, since the subject selection process took place in March 2023, the possibility of further exclusions of non-LD cases in the final selection is likely to be extremely low. Second, to obtain the incidence rate of LD in Jeju Province, participants were limited to patients with addresses in Jeju Province, in accordance with the reporting guidelines of the KDCA, which manages cases according to their address [12]. However, one LD-diagnosed case was included while residing in another region, although their address was in Jeju Province. Since such cases can occur in all local governments nationwide, it was not intentionally excluded from statistical comparison. Third, among the 119 selected subjects, the route of infection was unknown in 26 cases (21.8%). Therefore, we first defined nosocomial infections based on the epidemiological investigation and treated all others as non-nosocomial infections. Since it was difficult to distinguish between community-acquired and domestically-acquired infections in patients with an unknown route of infection, as pointed out in the guidelines of the KDCA [12], the 2 routes were grouped together and classified as non-nosocomial infections. In addition, since all selected cases were sporadic occurrences, 1 travel-related infection case was also classified as a non-nosocomial infection.
- In conclusion, the rapid increase in the number of LD cases reported in Jeju Province was probably due to the pattern of medical-care use among Jeju residents, which was focused on a particular medical institution. Concurrent and active implementation of the UAT by that medical institution to screen patients suspected of community-acquired pneumonia in accordance with their treatment guidelines reduced false negatives. Thus, rather than inferring that the LD incidence rate after 2020 actually increased as compared to rates before 2019, it is more likely that some cases were not reported before 2019 due to missed diagnoses. Patients with actual LD were actively diagnosed after 2020 due to the introduction of the FIA-UAT. To confirm this hypothesis, epidemiological studies comparing and analyzing UAT use and pneumonia-related patterns in medical centers of other local governments will be required in the future. Moreover, as the proportion of LD in community-acquired pneumonia is expected to be higher than previously thought, more active infection control for LD should be implemented.
Discussion
- The rapid increase in Legionnaires’ disease in Jeju Province since 2020 may be related to the patterns of medical use by Jeju residents, which focused on a specific medical institution. The clinical practice guidelines of that medical institution called for the active implementation of urine antigen tests to screen patients suspected of community-acquired pneumonia.
HIGHLIGHTS
-
Ethics Approval
This study was approved for exemption by the IRB of Jeju National University as a study utilizing secondary data (JJNU-IRB-2023-019).
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
This study was supported by the 2023 Education, Research, and Student Guidance grant funded by Jeju National University.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
Article information
-
Acknowledgements
- The authors would like to express their gratitude to Professor Young Ree Kim from the Department of Laboratory Medicine at Jeju National University Hospital and Ms. Kyung-Mi Kim, team leader of the Jeju Center for Infectious Disease Control and Prevention, for providing advice on study design and interpretation of the results.
| Characteristic | Total (n=119) | 2015–2019 (n=21) | 2020–2022 (n=98) | p |
|---|---|---|---|---|
| Age (y) | 74.53±14.84 | 63.81±16.12 | 76.83±13.57 | <0.001*** |
| Sex | 0.225 | |||
| Male | 77 (64.7) | 16 (76.2) | 61 (62.2) | |
| Female | 42 (35.3) | 5 (23.8) | 37 (37.8) | |
| Hospital | <0.05* | |||
| A | 102 (85.7) | 14 (66.7) | 88 (89.8) | |
| B | 3 (2.5) | 2 (9.5) | 1 (1.0) | |
| C | 10 (8.4) | 3 (14.3) | 7 (7.1) | |
| D | 2 (1.7) | 1 (4.8) | 1 (1.0) | |
| Other | 2 (1.7) | 1 (4.8) | 1 (1.0) | |
| Diagnosis | <0.05* | |||
| UAT | 91 (76.5) | 12 (57.1) | 79 (80.6) | |
| UAT+culture | 3 (2.5) | 0 (0) | 3 (3.1) | |
| UAT+PCR | 10 (8.4) | 3 (14.3) | 7 (7.1) | |
| PCR | 15 (12.6) | 6 (28.6) | 9 (9.2) | |
| Route | 0.817 | |||
| Nosocomial | 19 (16.0) | 3 (14.3) | 16 (16.3) | |
| Non-nosocomial | 100 (84.0) | 18 (85.7) | 82 (83.7) |
- 1. Graham FF, Finn N, White P, et al. Global perspective of Legionella infection in community-acquired pneumonia: a systematic review and meta-analysis of observational studies. Int J Environ Res Public Health 2022;19:1907. ArticlePubMedPMC
- 2. Wunderink RG, Waterer GW. Clinical practice: community-acquired pneumonia. N Engl J Med 2014;370:543−51.ArticlePubMed
- 3. Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect 2006;12 Suppl 3:12−24.ArticlePubMedPMC
- 4. Gramegna A, Sotgiu G, Di Pasquale M, et al. Atypical pathogens in hospitalized patients with community-acquired pneumonia: a worldwide perspective. BMC Infect Dis 2018;18:677. ArticlePubMedPMCPDF
- 5. Allgaier J, Lagu T, Haessler S, et al. Risk factors, management, and outcomes of legionella pneumonia in a large, nationally representative sample. Chest 2021;159:1782−92.ArticlePubMed
- 6. Prina E, Ranzani OT, Torres A. Community-acquired pneumonia. Lancet 2015;386:1097−108.ArticlePubMedPMC
- 7. Marchello C, Dale AP, Thai TN, et al. Prevalence of atypical pathogens in patients with cough and community-acquired pneumonia: a meta-analysis. Ann Fam Med 2016;14:552−66.ArticlePubMedPMC
- 8. Jespersen S, Sogaard OS, Fine MJ, et al. The relationship between diagnostic tests and case characteristics in Legionnaires’ disease. Scand J Infect Dis 2009;41:425−32.ArticlePubMed
- 9. Alarcon Falconi TM, Cruz MS, Naumova EN. The shift in seasonality of legionellosis in the USA. Epidemiol Infect 2018;146:1824−33.ArticlePubMedPMC
- 10. Fischer FB, Schmutz C, Gaia V, et al. Legionnaires’ disease on the rise in Switzerland: a denominator-based analysis of national diagnostic data, 2007-2016. Int J Environ Res Public Health 2020;17:7343. ArticlePubMedPMC
- 11. Leung YH, Lam CK, Cheung YY, et al. Epidemiology of Legionnaires’ disease, Hong Kong, China, 2005-2015. Emerg Infect Dis 2020;26:1695−702.ArticlePubMedPMC
- 12. Korea Disease Control and Prevention Agency (KDCA). Manual for control of mite/rodent-mediated infectious diseases [Internet]. KDCA; 2023 [cited 2023 Mar 15]. Available from: https://kdca.go.kr/board/board.es?mid=a20507020000&bid=0019. Korean.
- 13. Korea Disease Control and Prevention Agency (KDCA). Infectious disease homepage [Internet]. KDCA; 2023 [cited 2023 Feb 15] Available from: https://npt.kdca.go.kr/npt/biz/npp/ist/simple/simplePdStatsMain.do. Korean.
- 14. Mathieu E, Sodahlon Y. Epidemic investigation. Int Encycl Public Health 2017;518−29.Article
- 15. Burillo A, Pedro-Botet ML, Bouza E. Microbiology and epidemiology of Legionnaire’s disease. Infect Dis Clin North Am 2017;31:7−27.ArticlePubMed
- 16. Min J, Kim HW, Koo HK, et al. Impact of COVID-19 pandemic on the national PPM tuberculosis control project in Korea: the Korean PPM Monitoring Database between July 2019 and June 2020. J Korean Med Sci 2020;35:e388.ArticlePubMedPMCPDF
- 17. Birtles RJ, Harrison TG, Samuel D, et al. Evaluation of urinary antigen ELISA for diagnosing Legionella pneumophila serogroup 1 infection. J Clin Pathol 1990;43:685−90.ArticlePubMedPMC
- 18. Viasus D, Gaia V, Manzur-Barbur C, et al. Legionnaires’ disease: update on diagnosis and treatment. Infect Dis Ther 2022;11:973−86.ArticlePubMedPMCPDF
- 19. Wong AY, Johnsson AT, Iversen A, et al. Evaluation of four lateral flow assays for the detection of Legionella urinary antigen. Microorganisms 2021;9:493. ArticlePubMedPMC
- 20. Cunha BA, Burillo A, Bouza E. Legionnaires’ disease. Lancet 2016;387:376−85.ArticlePubMed
- 21. Joseph CA; European Working Group for Legionella Infections. Legionnaires’ disease in Europe 2000-2002. Epidemiol Infect 2004;132:417−24.ArticlePubMedPMC
- 22. Beaute J; The European Legionnaires’ Disease Surveillance Network. Legionnaires’ disease in Europe, 2011 to 2015. Euro Surveill 2017;22:30566. PubMedPMC
- 23. Regan CM, Syed Q, Mutton K, et al. A pseudo community outbreak of Legionnaires’ disease on Merseyside; implications for investigation of suspected clusters. J Epidemiol Community Health 2000;54:766−9.ArticlePubMedPMC
- 24. Centers for Disease Control and Prevention (CDC). Legionellosis: United States, 2000-2009. MMWR Morb Mortal Wkly Rep 2011;60:1083−6.PubMed
- 25. Diederen BM, Peeters MF. Evaluation of two new immunochromatographic assays (Rapid U Legionella antigen test and SD Bioline Legionella antigen test) for detection of Legionella pneumophila serogroup 1 antigen in urine. J Clin Microbiol 2006;44:2991−3.ArticlePubMedPMCPDF
- 26. Formica N, Yates M, Beers M, et al. The impact of diagnosis by legionella urinary antigen test on the epidemiology and outcomes of Legionnaires’ disease. Epidemiol Infect 2001;127:275−80.ArticlePubMedPMC
- 27. Lee MS, Oh JY, Kang CI, et al. Guideline for antibiotic use in adults with community-acquired pneumonia. Infect Chemother 2018;50:160−98.ArticlePubMedPMCPDF
- 28. Sopena N, Pedro-Botet L, Mateu L, et al. Community-acquired Legionella pneumonia in elderly patients: characteristics and outcome. J Am Geriatr Soc 2007;55:114−9.ArticlePubMed
- 29. Jorgensen CS, Uldum SA, Sorensen JF, et al. Evaluation of a new lateral flow test for detection of Streptococcus pneumoniae and Legionella pneumophila urinary antigen. J Microbiol Methods 2015;116:33−6.ArticlePubMed
- 30. Beraud L, Gervasoni K, Freydiere AM, et al. Comparison of Sofia Legionella FIA and BinaxNOW® Legionella urinary antigen card in two national reference centers. Eur J Clin Microbiol Infect Dis 2015;34:1803−7.ArticlePubMedPMCPDF
References
Figure & Data
References
Citations



 Cite
Cite