Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 15(1); 2024 > Article
-
Original Article
Living arrangements and metabolic syndrome: a national cross-sectional study in the Republic of Korea -
Junghyun Kim1
 , Aeree Sohn2
, Aeree Sohn2
-
Osong Public Health and Research Perspectives 2023;15(1):77-82.
DOI: https://doi.org/10.24171/j.phrp.2023.0036
Published online: September 20, 2023
1Department of Clinical Laboratory Science, Kyungbok University, Namyangju, Republic of Korea
2Department of Public Health, Sahmyook University, Seoul, Republic of Korea
- Correspondence to: Aeree Sohn Department of Public Health, Sahmyook University, 815 Hwarang-ro, Nowon-gu, Seoul, 01795, Republic of Korea E-mail: aeree@syu.ac.kr
© 2024 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 1,150 Views
- 46 Download
Abstract
-
Objectives
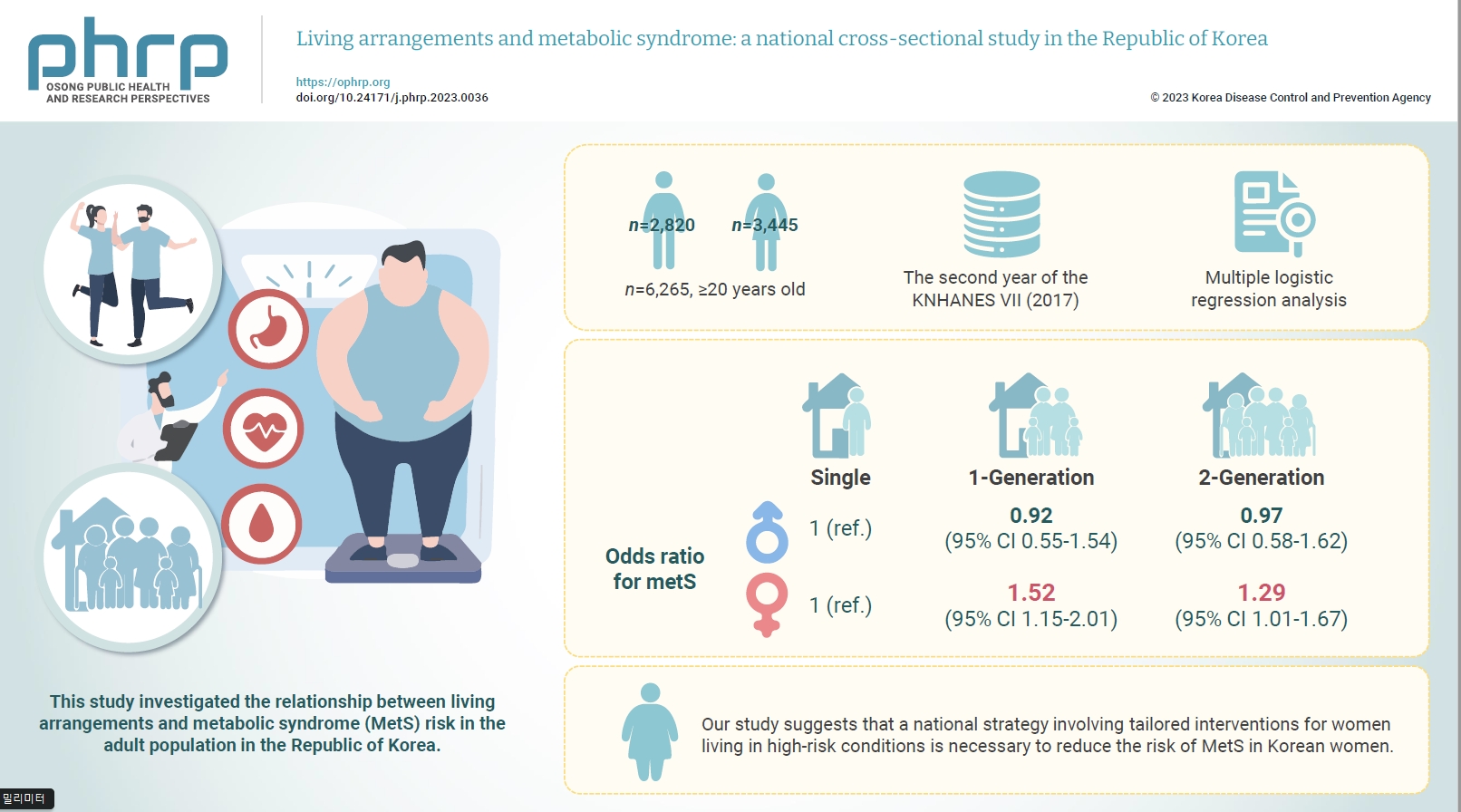
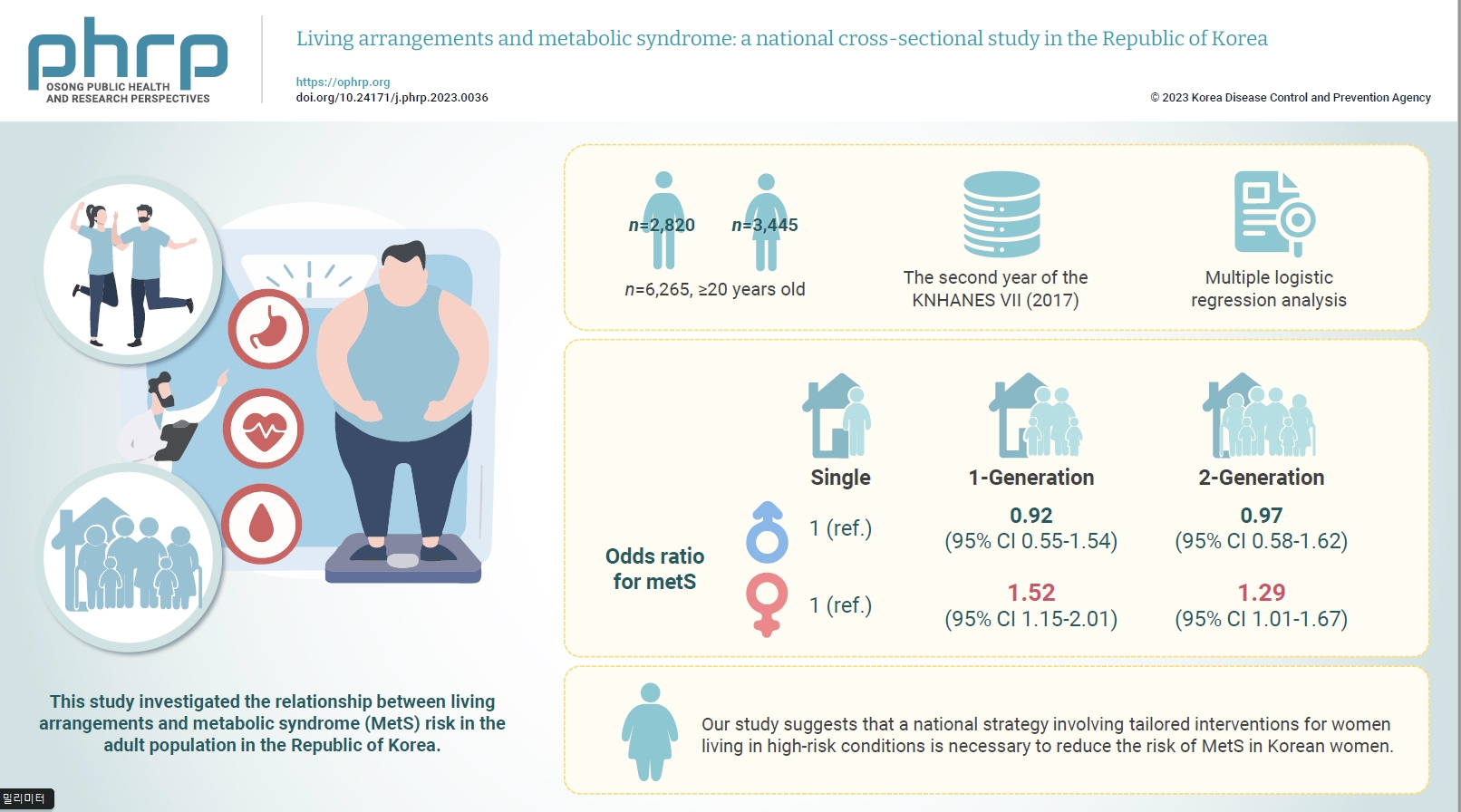
- This study investigated the relationship between living arrangements and metabolic syndrome (MetS) risk in the adult population in the Republic of Korea.
-
Methods
- The samples were derived from the data collected during the second year of the seventh Korea National Health and Nutrition Examination Survey. The study targeted a total of 6,265 adults who were aged 20 years and above, and multiple logistic regression analysis was conducted. Living arrangements were classified into 4 categories: single-person households, 1-generation households, 2-generation households, and other family types. MetS was identified by the presence of at least 3 out of the 5 National Cholesterol Education Program Adult Treatment Panel III criteria.
-
Results
- For men, the odds ratio (ORs) for MetS in 1- and 2-generation households, compared to single-person households, were 0.92 (95% confidence interval [CI], 0.55–1.54) and 0.97 (95% CI, 0.58–1.62), respectively. The OR for other types of households was 0.96 (95% CI, 0.79–1.17). For women, the OR for MetS in 1- and 2-generation households, compared to single-person households, were 1.52 (95% CI, 1.15–2.01) and 1.29 (95% CI, 1.01–1.67), respectively.
-
Conclusion
- Our study suggests that a national strategy involving tailored interventions for women living in high-risk conditions is necessary to reduce the risk of MetS in Korean women.
- Metabolic syndrome (MetS), which comprises a cluster of risk factors for cardiovascular and disease and all-cause mortality, is a global health problem [1]. In the Republic of Korea, the prevalence of MetS rose from 1.53% in 2008 to 3.19% in 2017 [2].
- Recent studies have reported that genetic variations and shared environmental factors contribute to the heritability of MetS, as evidenced by familial correlations [3]. Some research has explored the relationship between familial ties and environmental factors, including lifestyle changes, climate, geography, and migration [4]. The advantages and disadvantages of different living arrangements, such as living alone or living with children, have also been discussed [5]. From another angle, a connection has been observed between single-child families and cardio-metabolic risk factors [6]. There is a growing interest in the relationship between MetS, marital status, and living arrangements, with numerous studies investigating the link between social and economic conditions and MetS [7−9].
- From the standpoint of familial aggregation, the structure of a family has been identified as a significant predictive factor for maternal and paternal connections [10]. Marriage, in particular, encourages healthy behavior and bolsters mental health, thereby serving as a crucial source of social support that contributes to physical well-being. Furthermore, marriage can exert social control over lifestyle habits that may be detrimental to one's health [11−13].
- Due to shifts in economic structure and development, the traditional extended family structure in Korea has recently transitioned into a nuclear family structure. Consequently, the number of single-generation families has significantly increased [14,15]. These alterations in family structure are transforming adult lifestyles, thereby significantly influencing their healthcare issues [7,16,17].
- Previous studies have examined the associations of living arrangements with MetS [18−22] and psychological health [23−26]. Studies have also concentrated on the correlation between socioeconomic status and MetS in middle-aged and elderly people [5, 9,10,12,27]. Despite the numerous studies on MetS, there is a dearth of research examining the relationship between living arrangements and the incidence of MetS. Consequently, this study aims to investigate the connection between living arrangements and the risk of MetS in the Korean adult population, with a focus on gender differences. The data for this study was sourced from the Korea National Health and Nutrition Examination Surveys (KNHANES), conducted by the Korea Disease Control and Prevention Agency.
Introduction
- Study Participants
- This study utilized data from the second year of the KNHANES Ⅶ (2017), an annual survey that includes a health examination, health interview, and nutrition survey. The survey employs a stratified multistage cluster sampling method to draw a representative sample from the non-institutionalized civilian population of the Republic of Korea. Participants with missing data for at least 1 variable related to MetS were excluded from the study. Ultimately, the study included 6,265 participants. All participants provided informed consent to participate in the survey.
- Demographic Characteristics, Anthropometric Variables, and Living Arrangement
- For the health interview survey, we chose demographic variables such as age, gender, residential area, physical activity, alcohol consumption, smoking status, and self-perceived health condition. We selected education level and household income level as socioeconomic indicators. Additionally, we used body mass index (BMI) and waist circumference (WC) as anthropometric variables.
- The area of residence was categorized into rural and urban. The definition of a rural residence was provided in the health interview survey. Physical exercise was divided into non-exercise and regular exercise. The regular exercise category included individuals who exercised 3 or more times a week, with each session lasting more than 20 minutes. Alcohol consumption was split into 3 categories: non-drinkers, mild-moderate drinkers, and heavy drinkers. Individuals who consumed 3 or more drinks per day were classified as heavy drinkers, while those who consumed alcohol once or more a month were simply classified as drinkers. Smoking status was divided into 2 categories: non-smokers and current smokers. A current smoker was defined as an individual who had smoked 100 or more cigarettes and was still smoking. The non-smoker category included former smokers. Education level was divided into 4 categories: elementary school, middle school, high school, and university. Household income level was divided into 4 categories based on quartiles: lowest, middle-low, middle-high, and highest. WC was measured at the narrowest point between the lower border of the rib cage and the iliac crest. The formula for calculating BMI is as follows: weight (kg)/height squared (m2). Living arrangements were divided into 4 groups: (1) single-person households, adults living alone; (2) 1-generation households, adults living with a spouse; (3) 2-generation households, adults living with children; (4) others, adults living with grandparents and other relatives.
- Definitions of MetS
- According to the updated National Cholesterol Education Program Adult Treatment Panel III criteria, MetS is defined by the presence of 3 or more of the following 5 criteria: (1) a WC of 90 cm or more in men, or 80 cm or more in women, as per the International Obesity Task Force standards for the Asian-Pacific population; (2) a blood pressure (BP) reading of 130/85 mmHg or higher, or the use of antihypertensive medication; (3) a fasting blood glucose level of 100 mg/dL or higher, or the use of medication such as insulin or oral agents; (4) a triglyceride level of 150 mg/dL or higher, or the use of medication; and (5) a high-density lipoprotein (HDL) cholesterol level less than 40 mg/dL in men or less than 50 mg/dL in women, or the use of medication.
- Statistical Analysis
- We utilized the SAS survey procedure ver. 9.2 (SAS Institute Inc.) for our statistical analysis. To compare differences in anthropometric, laboratory, and demographic variables based on gender, we employed either the t-test or the chi-square test. We used the chi-square test to compare the prevalence of MetS and each of its components according to different living arrangements. Multiple logistic regression analyses were conducted to assess the risk of MetS as an independent variable related to living arrangements. In these analyses, the reference group for living arrangements was single-person households.
- Odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of MetS were calculated for different groups: single-person households, 1-generation households, 2-generation households, and others. We examined the changes in the OR for MetS risk in each living arrangement, adjusting for age and BMI in model 1. In model 2, we further adjusted for exercise, alcohol consumption, smoking habits, and self-reported health condition. Finally, in model 3, we adjusted for the covariates in model 2, as well as household income and education level. A 2-sided p-value of less than 0.05 was deemed statistically significant.
Materials and Methods
- Table 1 shows the general characteristics of the 6,265 study participants according to gender. The average age of men was 41 years, and that of women was 44 years. The average age for men was 41 years, while for women it was 44 years. The distribution of living arrangements for men was as follows: 2-generation households, 61.8%; 1-generation households, 28.0%; single-person households, 5.4%; and other arrangements, 4.8%. For women, the distribution was as follows: 2-generation households, 57.7%; 1-generation households, 28.4%; single-person households, 8.2%; and other arrangements, 5.7%. Both household income and education level were higher among men than women. Men also smoked and consumed alcohol much more frequently than women, despite exercising more regularly. Furthermore, men exhibited higher levels of BMI, WC, BP, fasting glucose, and triglyceride compared to women.
- Table 2 illustrates the univariate relationship between living arrangements and MetS, along with its components, differentiated by gender. The incidence of MetS in single-person households was 24.6% for men and 45.3% for women. In 1-generation households, the prevalence was 30.9% for men and 40.2% for women. In 2-generation households, the rates were 20.9% for men and 17.3% for women. In other types of households, the prevalence was 7.1% for men and 9.9% for women. For both genders, a larger living arrangement corresponded to a lower prevalence of MetS (p<0.001). The prevalence of each of the 5 MetS components showed a significant variation based on living arrangement (p<0.001). For men, a larger living arrangement was associated with a lower proportion of high BP, high blood glucose, high triglycerides, and low HDL cholesterol, with the exception of abdominal obesity. For women, a larger living arrangement corresponded to a lower proportion of abdominal obesity, high BP, high blood glucose, high triglycerides, and low HDL cholesterol (p<0.001).
- Table 3 depicts the ORs for MetS across different living arrangements, with the single-person household group serving as the reference. For men, the adjusted ORs for the 1-generation, 2-generation, and other categories compared to the single-person household were 0.92 (95% CI, 0.55–1.54), 0.97 (95% CI, 0.58–1.62), and 0.96 (95% CI, 0.79–1.17), respectively, in model 3. For women, the adjusted OR for the 1-generation category compared to single-person households was 1.34 (95% CI, 1.07–1.69; p<0.001) in model 1. When additional behavioral risk factors (alcohol, smoking, and exercise) were adjusted for, the OR increased to 1.44 (95% CI, 1.14–1.82; p<0.001) in model 2. Further adjustment for socioeconomic factors (household income and education level) resulted in an OR of 1.52 (95% CI, 1.15–2.01; p<0.001) in model 3. The OR for MetS in the 2-generation category compared to single-person households was 1.29 (95% CI, 1.01–1.67; p<0.001) after adjusting for all components. However, the OR for the “other” category compared to single-person households was not statistically significant.
Results
- This study investigated the association between living arrangements and the risk of MetS in the adult population of the Republic of Korea, using data from the second year of the KNHANES Ⅶ. The findings indicated a downward trend in the adjusted ORs for 1- and 2-generation households, as well as other living arrangements, compared to single-person households among men. Conversely, there was an upward trend in the adjusted ORs for 1- and 2-generation households, and other living arrangements, compared to single-person households among women.
- Significant gender differences were revealed in the association between living arrangements and the risk of MetS. A similar gender-specific association was found in another Chinese study, which attributed the risk of MetS to various factors such as age, physical activity, and education level. Some of these findings align with ours, even though living arrangements were not considered in that study [28]. Previous research has reported familial correlations with MetS, which supports our findings. These studies suggest that both shared genetic and environmental factors contribute to the risk of MetS, reinforcing the importance of considering living arrangements [3].
- There is compelling evidence from a previous cohort study suggesting a gender difference in the relationship between markers of MetS and an elevated risk of cardiovascular disease [29]. In our study, the higher prevalence of MetS in women than in men could be partially explained by another study’s findings, which indicated an increase in MetS in women following menopause [19].
- Our study showed that a 2-generation living arrangement appeared to reduce the risk of MetS more significantly in women than a 1-generation arrangement. To the best of our knowledge, this is the first study to investigate the relationship between MetS and different living situations, with a specific focus on gender differences.
- Living in a household with a spouse and children has been reported to provide health benefits [5]. These findings have been partially attributed to the shared environment and familial correlations. More specifically, in men, the risk of MetS was found to be higher in single-person households compared to those living with a spouse in a 1-generation family. However, this effect was moderated by factors such as income and education level. In the case of women, obesity was identified as a significant issue, with socio-demographic factors and lifestyle behaviors serving as explanatory variables [30].
- Our study’s findings regarding gender differences in MetS are corroborated by another study, which also identified gender disparities in MetS and its associated factors in Taiwan [10].
- Our study has several strengths. Firstly, we incorporated a variety of living arrangements to reflect the recent shifts in living trends. Secondly, we noted gender-based differences, where distinct lifestyles emerged as the pivotal factor linking MetS to various living arrangements.
- In conclusion, this study determined that individuals living with family members, particularly with a spouse, have an increased risk of MetS compared to those living alone. This risk is especially pronounced among women. Our study indicates that it is necessary to implement a national strategy involving interventions tailored for women with high-risk living conditions to reduce the risk of MetS in Korean women. Additional prospective studies should be conducted to further develop these findings and investigate the mechanisms that promote sustainable strategies and management of MetS.
Discussion
- • This study investigated the relationship between living arrangements and metabolic syndrome risk in the adult population in the Republic of Korea, using the Korea National Health and Nutrition Examination Surveys conducted by the Korea Disease Control and Prevention Agency.
- • This study suggests that a national strategy involving customized interventions for women with risky living arrangements should be pursued to decrease the risk of metabolic syndrome in Korean women.
HIGHLIGHTS
-
Ethics Approval
Not applicable.
-
Conflicts of Interest
The authors have no conflicts of interest to declare. Aeree Sohn was not involved in the editorial process or review of this manuscript, despite being a member of the Editorial Board of the Osong Public Health and Research Perspectives.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
Article information
| Characteristic | Man (n=2,820) | Woman (n=3,445) | pa) |
|---|---|---|---|
| Age (y) | 41.6±0.3 | 44.4±0.3 | <0.001 |
| Residence area | 0.35 | ||
| Rural | 19.8 (1.7) | 19.3 (1.7) | |
| Exercise | <0.001 | ||
| Yes | 24.1 (0.6) | 16.9 (0.5) | |
| Alcohol drinking | <0.001 | ||
| Non-drinker | 23.1 (0.6) | 38.9 (0.6) | |
| Mild-to-moderate drinker | 61.4 (0.7) | 59 (0.6) | |
| Heavy drinker | 15.5 (0.5) | 2 (0.2) | |
| Smoking | <0.001 | ||
| Current smoker | 57.8 (0.7) | 8.7 (0.3) | |
| Self-health condition | <0.001 | ||
| Good | 39.9 (1.3) | 40.1 (1.4) | |
| Moderate | 35.3 (0.7) | 35.2 (0.8) | |
| Bad | 24.8 (1.3) | 24.7 (1.3) | |
| Education | <0.001 | ||
| Elementary school or less | 17.8 (0.5) | 28 (0.7) | |
| Middle school | 13.6 (0.5) | 13.2 (0.5) | |
| High school | 37 (0.7) | 32.9 (0.7) | |
| College and higher | 31.5 (0.7) | 25.9 (0.7) | |
| Income | <0.001 | ||
| Lowest | 13.2 (0.6) | 17 (0.6) | |
| Medium-lowest | 26.8 (0.8) | 28.2 (0.8) | |
| Medium-highest | 30.7 (0.8) | 28.2 (0.7) | |
| Highest | 29.3 (0.9) | 26.7 (0.8) | |
| Living arrangement | <0.001 | ||
| Single | 5.4 (0.7) | 8.2 (0.7) | |
| One-generation | 28.0 (0.6) | 28.4 (0.6) | |
| Two-generation | 61.8 (0.8) | 57.7 (0.8) | |
| Others | 4.8 (0.4) | 5.7 (0.3) | |
| BMI (kg/m2) | 23.7±0.1 | 22.9±0.1 | <0.001 |
| WC (cm) | 82.4±0.2 | 76.7±0.2 | <0.001 |
| BP (mmHg) | 123.8±16.5 | 122.4±18.3 | <0.001 |
| Fasting glucose (mg/dL) | 105.5±27.2 | 100.9±24.5 | <0.001 |
| TG (mg/dL) | 163.9±133.5 | 131±88.2 | <0.001 |
| HDL cholesterol (mg/dL) | 45.9±11.2 | 50.3±11.7 | <0.001 |
| Variable | Single | One-generation | Two-generation | Others | p for trenda) |
|---|---|---|---|---|---|
| Man | |||||
| Metabolic syndrome | 24.6 (2.7) | 30.9 (1.3) | 20.9 (0.7) | 7.1 (0.7) | <0.001 |
| Abdominal obesity | 22.5 (3.1) | 25.6 (1.3) | 21.3 (0.8) | 5.5 (0.9) | 0.0613 |
| High blood pressure | 40.4 (3.3) | 52.5 (1.5) | 34.7 (0.9) | 8.5 (1.1) | <0.001 |
| High fasting glucose | 31.8 (3.1) | 36.5 (1.3) | 24.4 (0.8) | 10.1 (0.8) | <0.001 |
| High triglyceride | 41.1 (3.4) | 39.7 (1.4) | 33.1 (0.8) | 7.9 (1.1) | <0.001 |
| Low HDL cholesterol | 22.6 (3.0) | 26.1 (1.4) | 18.8 (0.7) | 6.1 (0.9) | <0.001 |
| Woman | |||||
| Metabolic syndrome | 45.3 (2.4) | 40.2 (1.3) | 17.3 (0.6) | 9.9 (0.5) | <0.001 |
| Abdominal obesity | 51.0 (2.6) | 50.2 (1.5) | 30.9 (0.7) | 8.2 (0.6) | <0.001 |
| High blood pressure | 54.6 (2.6) | 48.0 (1.5) | 20.1 (0.7) | 8.6 (0.4) | <0.001 |
| High fasting glucose | 35.4 (2.2) | 30.6 (1.2) | 15.4 (0.6) | 12.7 (0.6) | <0.001 |
| High triglyceride | 35.1 (2.3) | 34.9 (1.3) | 18.5 (0.6) | 8.4 (0.7) | <0.001 |
| Low HDL cholesterol | 46.1 (2.4) | 46.9 (1.3) | 32.6 (0.8) | 12.2 (1.1) | <0.001 |
| Variable | Model 1a) | Model 2b) | Model 3c) |
|---|---|---|---|
| Man | |||
| Single | 1.00 | 1.00 | 1.00 |
| One-generation | 0.88 (0.57–1.36) | 1.02 (0.65–1.60) | 0.92 (0.55–1.54) |
| Two-generation | 1.04 (0.70–1.56) | 1.13 (0.74–1.73) | 0.97 (0.58–1.62) |
| Others | 1.01 (0.82–1.22) | 0.96 (0.79–1.17) | 0.96 (0.79–1.17) |
| Woman | |||
| Single | 1.00 | 1.00 | 1.00 |
| One-generation | 1.34 (1.07–1.69) | 1.44 (1.14–1.82) | 1.52 (1.15–2.01) |
| Two-generation | 1.10 (0.86–1.40) | 1.33 (0.98–1.79) | 1.29 (1.01–1.67) |
| Others | 1.04 (0.89–1.22) | 1.03 (0.88–1.22) | 1.06 (0.90–1.25) |
- 1. Yu WW, Randhawa AK, Blair SN, et al. Age- and sex- specific all-cause mortality risk greatest in metabolic syndrome combinations with elevated blood pressure from 7 U.S. cohorts. PLoS One 2019;14:e0218307.ArticlePubMedPMC
- 2. Park SI, Suh J, Lee HS, et al. Ten-year trends of metabolic syndrome prevalence and nutrient intake among Korean children and adolescents: a population-based study. Yonsei Med J 2021;62:344−51.ArticlePubMedPMCPDF
- 3. Fernandez-Rhodes L, Howard AG, Tao R, et al. Characterization of the contribution of shared environmental and genetic factors to metabolic syndrome methylation heritability and familial correlations. BMC Genet 2018;19(Suppl 1):69.
- 4. Kesebir S. Reciprocal roots of metabolic syndrome and affective disorder: temperamental, familial and environmental factors such as climate, geography, migration and changeable life styles. Ment Health Fam Med 2018;14:772−4.
- 5. Li LW, Zhang J, Liang J. Health among the oldest-old in China: which living arrangements make a difference? Soc Sci Med 2009;68:220−7.ArticlePubMedPMC
- 6. Kelishadi R, Qorbani M, Rezaei F, et al. Is single-child family associated with cardio-metabolic risk factors: the CASPIAN-V study. BMC Cardiovasc Disord 2018;18:109. ArticlePubMedPMCPDF
- 7. Boldis BV, San Sebastian M, Gustafsson PE. Unsafe and unequal: a decomposition analysis of income inequalities in fear of crime in northern Sweden. Int J Equity Health 2018;17:110. ArticlePubMedPMCPDF
- 8. Chaiyasoot K, Sarasak R, Pheungruang B, et al. Evaluation of a 12-week lifestyle education intervention with or without partial meal replacement in Thai adults with obesity and metabolic syndrome: a randomised trial. Nutr Diabetes 2018;8:23. ArticlePubMedPMCPDF
- 9. Wickrama KA, O'Neal CW, Neppl TK. Midlife family economic hardship and later life cardiometabolic health: the protective role of marital integration. Gerontologist 2019;59:892−901.ArticlePubMedPMC
- 10. Liu CC, Chang HT, Chiang SC, et al. Sex differences in relationships between metabolic syndrome components and factors associated with health-related quality of life in middle-aged adults living in the community: a cross-sectional study in Taiwan. Health Qual Life Outcomes 2018;16:76. ArticlePubMedPMCPDF
- 11. Rueda S, Artazcoz L. Gender inequality in health among elderly people in a combined framework of socioeconomic position, family characteristics and social support. Ageing Soc 2009;29:625−47.Article
- 12. Tak YJ, Kim YJ, Lee SY, et al. Health care behavior of people 60 years and older in Korea according to family type and sociodemographic factors: the 5th Korea National Health and Nutrition Examination Survey. J Korean Geriatr Soc 2013;17:7−17.Article
- 13. Park E, Choi SJ, Lee HY. The prevalence of metabolic syndrome and related risk factors based on the KNHANES V 2010. J Agric Med Community Health 2013;38:1−13.Article
- 14. Lai KP, Chung YT, Li R, et al. Bisphenol A alters gut microbiome: comparative metagenomics analysis. Environ Pollut 2016;218:923−30.ArticlePubMed
- 15. Cho DY, Koo JW. Differences in metabolic syndrome prevalence by employment type and sex. Int J Environ Res Public Health 2018;15:1798. ArticlePubMedPMC
- 16. Damiri B, Abualsoud MS, Samara AM, et al. Metabolic syndrome among overweight and obese adults in Palestinian refugee camps. Diabetol Metab Syndr 2018;10:34. ArticlePubMedPMCPDF
- 17. Gentile C, Ditto B, Deschamps A, et al. Parasympathetic response patterns are associated with metabolic syndrome among older women but not men. Ann Behav Med 2019;53:515−26.ArticlePubMedPMC
- 18. Nam GE, Kim SM, Han K, et al. Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med 2018;15:e1002640.ArticlePubMedPMC
- 19. Zhu B, Zhang L, Cheng XP, et al. The association between metabolic syndrome and asymptomatic carotid artery stenosis in menopausal women: a cross-sectional study in a Chinese population. Ther Clin Risk Manag 2018;14:2183−8.ArticlePubMedPMCPDF
- 20. Cho KI, Sakuma I, Sohn IS, et al. Inflammatory and metabolic mechanisms underlying the calcific aortic valve disease. Atherosclerosis 2018;277:60−5.ArticlePubMed
- 21. Golabi P, Otgonsuren M, de Avila L, et al. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine (Baltimore) 2018;97:e0214.ArticlePubMedPMC
- 22. Yoon H, Gi MY, Cha JA, et al. The association between the metabolic syndrome and metabolic syndrome score and pulmonary function in non-smoking adults. Diab Vasc Dis Res 2018;15:131−8.ArticlePubMedPDF
- 23. Silverstein M, Cong Z, Li S. Intergenerational transfers and living arrangements of older people in rural China: consequences for psychological well-being. J Gerontol B Psychol Sci Soc Sci 2006;61:S256−66.ArticlePubMed
- 24. Taqui AM, Itrat A, Qidwai W, et al. Depression in the elderly: does family system play a role?: a cross-sectional study. BMC Psychiatry 2007;7:57. ArticlePubMedPMCPDF
- 25. Okabayashi H, Liang J, Krause N, et al. Mental health among older adults in Japan: do sources of social support and negative interaction make a difference? Soc Sci Med 2004;59:2259−70.ArticlePubMed
- 26. Jeon GS, Jang SN, Kim DS, et al. Widowhood and depressive symptoms among Korean elders: the role of social ties. J Gerontol B Psychol Sci Soc Sci 2013;68:963−73.ArticlePubMed
- 27. Kilpi F, Konttinen H, Silventoinen K, et al. Living arrangements as determinants of myocardial infarction incidence and survival: a prospective register study of over 300,000 Finnish men and women. Soc Sci Med 2015;133:93−100.ArticlePubMed
- 28. Li Y, Zhao L, Yu D, et al. Metabolic syndrome prevalence and its risk factors among adults in China: a nationally representative cross-sectional study. PLoS One 2018;13:e0199293.ArticlePubMedPMC
- 29. Awoyemi A, Troseid M, Arnesen H, et al. Markers of metabolic endotoxemia as related to metabolic syndrome in an elderly male population at high cardiovascular risk: a cross-sectional study. Diabetol Metab Syndr 2018;10:59. ArticlePubMedPMCPDF
- 30. Hong SA, Peltzer K, Lwin KT, et al. The prevalence of underweight, overweight and obesity and their related socio-demographic and lifestyle factors among adult women in Myanmar, 2015-16. PLoS One 2018;13:e0194454.ArticlePubMedPMC
References
Figure & Data
References
Citations



 Cite
Cite