Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(6); 2023 > Article
-
Review Article
Global prevalence of enterobiasis in young children over the past 20 years: a systematic review and meta-analysis -
Elham Kia Lashaki1
 , Azadeh Mizani2
, Azadeh Mizani2 , Seyed Abdollah Hosseini3
, Seyed Abdollah Hosseini3 , Bentolhoda Habibi4
, Bentolhoda Habibi4 , Khadijeh Taherkhani5
, Khadijeh Taherkhani5 , Amir Javadi6
, Amir Javadi6 , AliReza Taremiha7
, AliReza Taremiha7 , Samira Dodangeh8
, Samira Dodangeh8
-
Osong Public Health and Research Perspectives 2023;14(6):441-450.
DOI: https://doi.org/10.24171/j.phrp.2023.0204
Published online: December 28, 2023
1Department of Parasitology and Mycology, Faculty of Medical sciences, Tonekabon Branch, Islamic Azad University, Tonekabon, Iran
2Department of Parasitology, Pasteur Institute of Iran, Tehran, Iran
3Toxoplasmosis Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran
4Student Research Committee, Mazandaran University of Medical Science, Sari, Iran
5Dental Caries Prevention Research Center, Qazvin University of Medical Sciences, Qazvin, Iran
6Department of Social Medicine, Qazvin University of Medical Sciences, Qazvin, Iran
7Clinical Research Development Unit, Booalisina Hospital, Qazvin University of Medical Sciences, Qazvin, Iran
8Children Growth Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin University of Medical Sciences, Qazvin, Iran
- Corresponding author: Samira Dodangeh Children Growth Research Center, Research Institute for Prevention of Non-Communicable Diseases, Qazvin University of Medical Sciences, Quds Children’s Hospital, Valiasr Square, Shahid Beheshti Boulevard, First East Palestine, Qazvin, Iran E-mail: sdodangeh@ymail.com
© 2024 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 987 Views
- 54 Download
Abstract
- Parasitic infections are the most common diseases worldwide, and enterobiasis is a common parasitic infection in children. Various studies have reported on the prevalence of Enterobius vermicularis in different regions of the world. However, no study has gathered and analyzed this data systematically. Our systematic review and meta-analysis investigated the overall prevalence of E. vermicularis among children globally. Data were extracted from 4 available databases for studies published from January 2002 to April 2022. The quality of the included studies was scored based on the standard Strengthening the Reporting of Observational Studies in Epidemiology. A random-effect model was chosen to calculate the pooled prevalence and corresponding 95% confidence interval (CI) according to the degree of heterogeneity in the included studies. Thus, 40 publications (42 data sets) that included 3,279 children with enterobiasis met all criteria and were included in the analysis. The meta-analysis showed that heterogeneity among the included studies was high (Q=4,399.35, I2=99.96%; df=41; p<0.001). The pooled global prevalence of enterobiasis among the studied children was 12.9% (95% CI, 8.2%–17.7%). Our systematic review and meta-analysis estimated that, for the past 20 years, 12.9% of children around the world have been infected with E. vermicularis.
- Enterobiasis or oxyuriasis, a nematode infection caused by Enterobius vermicularis (E. vermicularis, pinworm, oxyure) is common among children and their family members [1]. E. vermicularis is a cosmopolitan parasite and one of the most common parasitic infections in many countries [2]. The World Health Organization reported that the prevalence of enterobiasis in children is between 4% and 28% [3]. It has been estimated that approximately 200 million people are infected worldwide, and over 30% of cases are children aged 5 to 10 years [4]. The prevalence of oxyuriasis among children has been reported as 2.5% to 45% in Latin America [5,6], 18% in Norway [7], 18.5% in the Republic of Korea [8], 17.2% in Iran [9], and 2.9% in north-central Ethiopia [10].
- Some patients with enterobiasis are asymptomatic, while others, especially children, may show symptoms such as perianal pruritus, restlessness, loss of appetite, malnutrition, anemia, insomnia, and irritability. Ectopic enterobiasis can penetrate the kidneys and fallopian tubes, leading to severe health disorders and even death [11,12].
- Although there are multiple ways to transmit enterobiasis, including the fecal-oral route, inhalation, auto-infection, and retrograde infection [13], the main route of transmission for E. vermicularis is direct contact between infected and uninfected individuals. Therefore, children in crowded environments such as kindergartens, schools, orphanages, and mental institutions are most susceptible to this infection [14]. The prevalence of this infection is mainly related to public health and personal hygiene [9]. Therefore, surveying enterobiasis infections in children can help us assess personal, familial, and social health status. The identification and prevention of pinworm transmission among children can promote infection control and benefit the health of children and the community [9].
- There are many publications regarding the prevalence of E. vermicularis infection among children. However, there are no comprehensive studies describing the status of enterobiasis infection in children globally. In the present systematic review and meta-analysis, we investigated the global pooled prevalence of E. vermicularis among young children.
Introduction
- Design
- This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines [15]. It also followed recommendations from the Cochrane Collaboration Handbook of Systematic Reviews [15] for the systematic, transparent, and reproducible investigation of scientific evidence.
- Search Strategy
- Two independent investigators (S.D., E.K.L.) searched 4 international databases (PubMed, Scopus, Science Direct, and ProQuest) for published studies that investigated the prevalence of E. vermicularis in children. English language publications between 2002 and 2022, with mesh terms (“Enterobius vermicularis” OR E. vermicularis OR Oxyuris OR pinworm OR roundworm OR seatworm) AND (Prevalence OR Rate) AND (Children OR Preschool OR Kindergarten), were collected. In addition, the reference lists of the identified articles were manually searched. We included studies conducted in the last 2 decades (01 January 2002 to 24 April 2022), published in English, and relevant to the aim of the study.
- Eligibility Criteria
- The eligibility criteria for the study were determined using the population, intervention, comparison, outcome, and study classification design (Table 1). The inclusion criteria were (1) cross-sectional studies that estimated the positive rate of E. vermicularis in children, (2) studies published online between January 2002 and April 2022, (3) original research papers, (4) studies published in English, (5) articles with full text, (6) studies that provided the total sample size and positive samples, and (7) studies that had a clear test method. Articles that did not meet these criteria were excluded. In addition, we contacted the corresponding authors to obtain more information if the data was incomplete. Reviews, case reports, letters to editors, and commentaries were not included in the study, but were used to enhance the search sensitivity to include any missed studies.
- Study Selection
- The 2-step study selection process included a title/abstract reading phase and a full-text reading phase. All search records were imported to EndNote software ver. X7.0.1 (Clarivate). All duplicates and irrelevant papers were excluded after the screening of titles/abstracts by 2 independent reviewers (S.D., E.K.L.). Any discrepancies were resolved by a third reviewer (S.A.H.). In the second phase, the full-text articles were downloaded and meticulously evaluated and discussed with 2 other reviewers and, if necessary, a third reviewer was consulted. Study selection was performed in accordance with the PRISMA flowchart.
- Data Extraction
- The data in each study were extracted independently by 2 reviewers (S.D., E.K.), using Microsoft Excel ver. 16.39 (Microsoft Corp.). Discrepancies were resolved by a third reviewer (A.M.). We included information on title, first author, year of publication, continent and country, sample size, number of positive samples, subject age and gender, and diagnostic method.
- Quality Assessment
- The quality of the included studies was scored using the standard Strengthening the Reporting of Observational Studies in Epidemiology (STROBE). A STROBE score of 25.6 to 34 indicated a high-quality study, and studies with scores of 16.6 to 25.5 and ≤16.5 were considered moderate and low quality, respectively. The articles included in our meta-analysis were deemed to have acceptable quality.
- Data Analysis
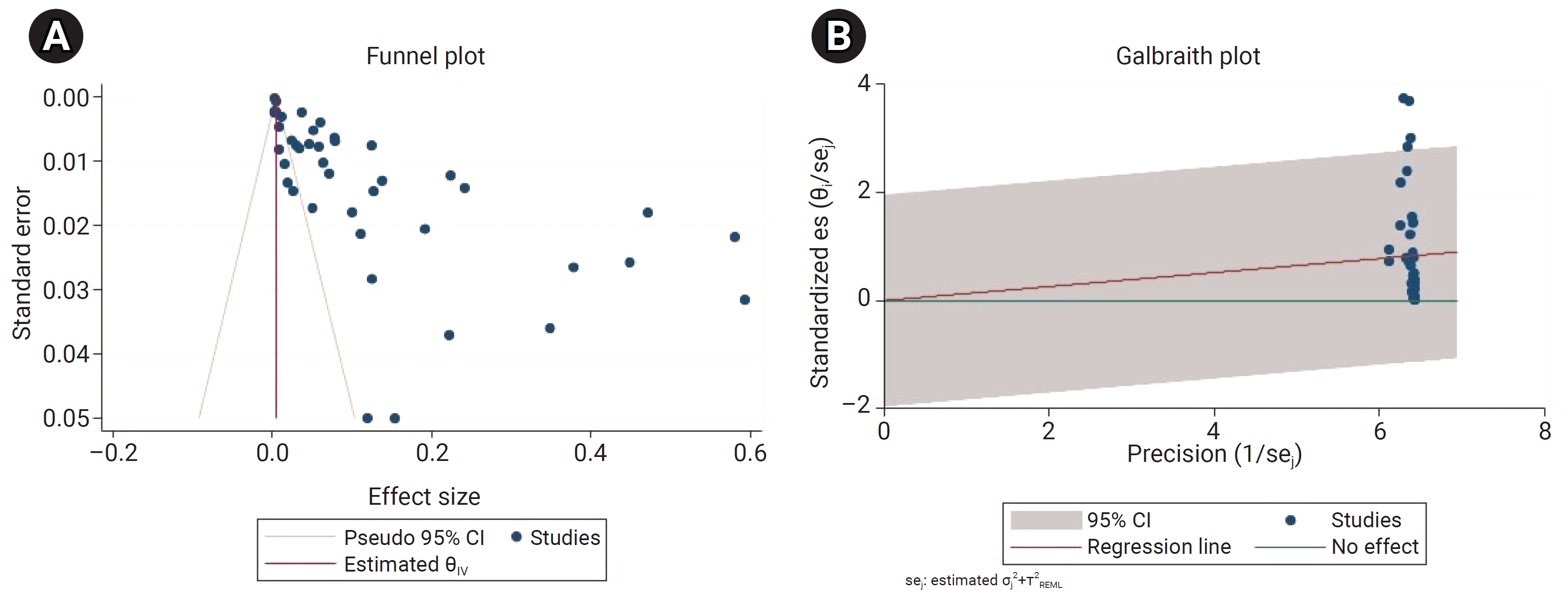
- We used Stata ver. 15.0 (Stata Corp.) for all statistical analyses. The heterogeneity among studies was calculated using both the Cochran Q test and the I2 statistic, with a cutoff at 50% to define a statistically significant degree of heterogeneity. The random-effect model was chosen according to the degree of heterogeneity in the included studies to calculate the pooled prevalence and corresponding 95% confidence interval (CI). To investigate the effect of different variables on heterogeneity, we did a subgroup analysis stratifying participants based on continent, age group, and publication decade. In addition, a funnel plot and an Egger test were used to assess the publication bias of selected studies.
- Ethics Approval
- This study received approval from the Qazvin University of Medical Sciences Ethical Committee Iran under the contract no. IR.QUMS.REC.1401.291.
Materials and Methods
- A flow chart of the study search and selection process for inclusion is shown in Figure 1. In our study, a total of 3,508 publications were searched. After removing duplicate articles, the titles and abstracts of the remaining 3,097 publications were screened. Finally, a total of 117 articles were selected for full paper review, of which 40 publications (42 data sets) fulfilled all criteria and were suitable for inclusion in the analysis. Among 60,176 children surveyed in these 40 publications, 3,279 children had enterobiasis.
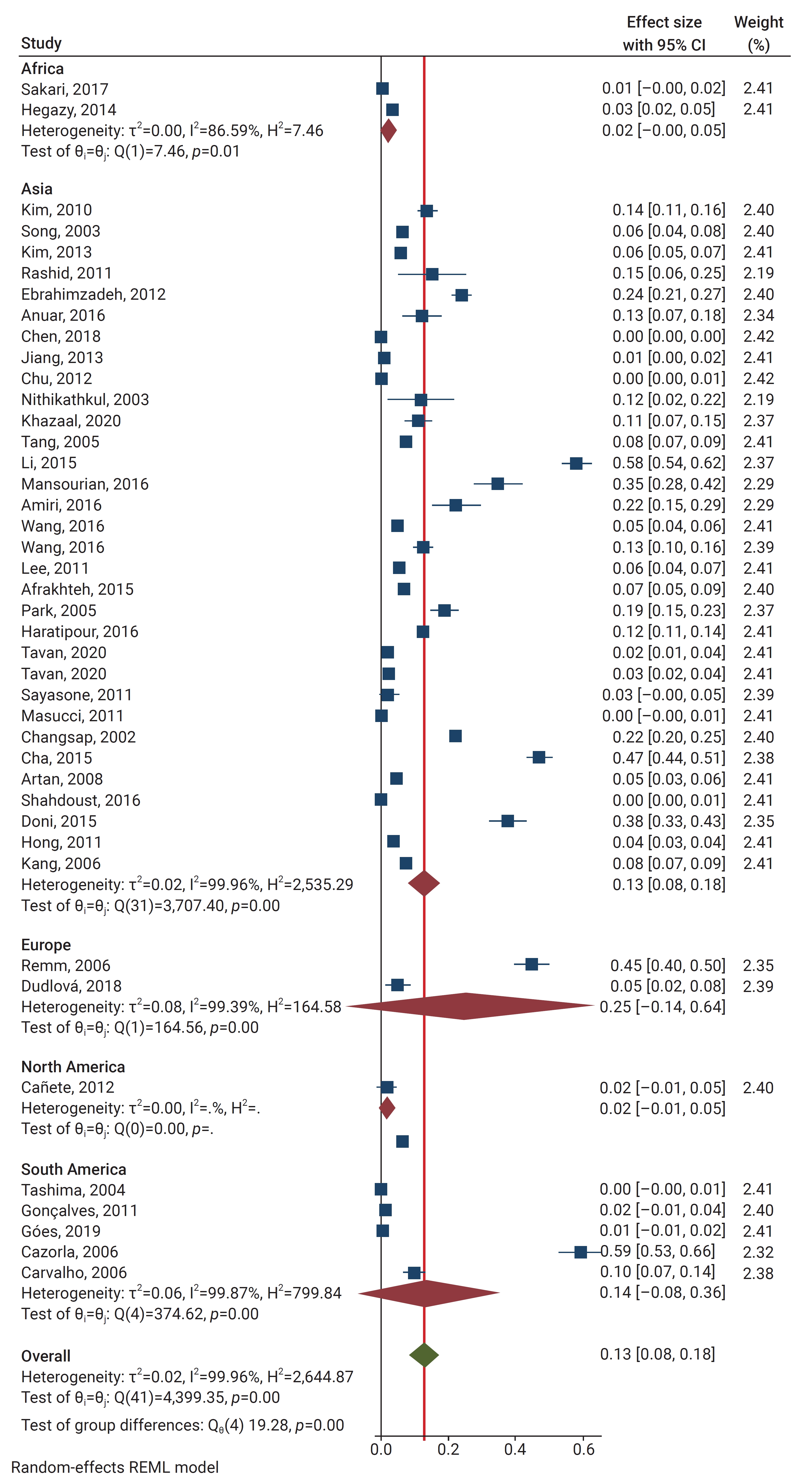
- Most of the included publications originated in Asia (32 out of 42, 76.2%). Five were from South America (11.9%), 2 from Africa (4.8%), 2 from Europe (4.8%), and 1 from North America (2.4%). Among the countries, Iran and The Republic of Korea had the most studies with 8 (19.0%) and 7 (16.7%) studies, respectively. The main characteristics of the included studies are summarized in Table 2 [8,11,16–53].
- Among the included studies, 14 investigated oxyuriasis in kindergarten children, 14 investigated preschool populations, and the remaining 7 studies investigated oxyuriasis in general child populations. Four were studies of children referred to medical clinics. Two studies were based on primary school children, and 1 studied child in a random population.
- The diagnostic methods used to detect E. vermicularis infection across studies were: cellophane tape swab (33 articles), cellophane anal swab (1 article), formalin-ethyl acetate concentration (3 articles), cellophane tape and formalin-ether concentration (1 article), microscopic formalin-ether concentration (1 article), the Lutz method (1 article), the Kato-Katz-direct smears (1 article), and the Kato-Katz method (1 article).
- The meta-analysis showed that heterogeneity among the included studies was very high (Q=4,399.35; I2=99.96%; df=41; p<0.001). Since the heterogeneity was significant, a random-effects model was used to estimate the pooled prevalence of enterobiasis among kindergarten and preschool children globally. In addition, the funnel plot and bias coefficient diagram did not show the presence of publication bias (b=–1.25; 95% CI, –0.0002 to 4.69; p=0.21) (Figure 2).
- Using the random effect method, the pooled global prevalence of enterobiasis among the children studied was 12.9% (95% CI, 8.2%–17.7%). The global pooled and weighted prevalence of enterobiasis among children based on geographic location was as follows (Figure 3): Europe 24.9% (95% CI, 0%–64%), South America 14.3% (95% CI, 0%–36.4%), Asia 13% (95% CI, 8.1%–17.9%), Africa 2% (95% CI, 0%–4.5%), and North America 1.9% (95% CI, 0%–4.6%).
- According to our meta-analysis, the primary school group exhibited the highest pooled prevalence of E. vermicularis infection, with a prevalence rate of 34.7% (95% CI, 10.5%–59%), followed by 20.2% (95% CI, 4.9%–35.5%) in the general child population group, 14.1% (95% CI, 0%–34.7%) in children referred to medical clinics, 11.2% (95% CI, 5.9%–16.5%) in kindergarten children, 8.2% (95% CI, 0.6%–15.8%) in preschool children, and 2.6% (95% CI, 0%–5.5%) in the random population group.
Results
- To the best of our knowledge, this is the first systematic review and meta-analysis to pool the global prevalence of enterobiasis in children. The current review included studies published between 2002 and 2022 that reported on the epidemiology of E. vermicularis infection in children worldwide. According to our review results, the global pooled estimated of children infected with enterobiasis was 12.9 %.
- Several regional studies on the prevalence of Enterobius infection have been conducted [4], showing enterobiasis prevalence among school children as follows: approximately 55% in China, 8.8% in Thailand, 47.2% in Myanmar, 4.4% in the Republic of Korea, and 19.3% in Kyrgyzstan (Asia); 26.3% in Tanzania, 1.7% in Angola, and 11.7% in Nigeria (Africa); 35% in Chile and 19% in Argentina (South America); and 17.4% in Germany.
- Pinworm infection is transmitted through direct contact with infected persons or objects. The worm is transmitted through ingestion of eggs, from the anus to the finger, fingernails, or hands when a patient scratches the perianal area where the gravid female worms emerge and deposit eggs [54]. Indirect aerosol transmission has also occurred in humans; the microscopic eggs can be released in the air and inhaled with dust [55]. Socioeconomic status, personal hygiene habits, and the environment are also important factors in the spread or prevention of E. vermicularis infection [56], contributing to its significance as a public health problem.
- Our data revealed a higher prevalence of E. vermicularis in younger children who lacked knowledge and understanding of this infection, its risk factors, and prevention. The major risk factors for enterobiasis include the personal hygiene habits of children, such as thumb-sucking and putting toys into their mouths; the overcrowded conditions in schools, kindergartens, and childcare centers; and inadequate sanitation. The risk factors for pinworm infection may vary in different countries [9,54]. According to a study conducted in Yemen, war led to increased pollution in the water and food supplies and worsened sanitary disposal systems and housing. These circumstances, along with a lack of heath awareness by the majority of parents and affected children were the main risk factors for disease [4].
- The diagnosis of enterobiasis is based on finding adult worms or eggs using the scotch tape technique or by stool examination, which is less sensitive. The scotch tape technique is the gold standard method for detection of enterobiasis in children because it is practical, easy, and inexpensive [13,57]. The fact that the worm’s eggs are sticky and adhere to the perianal skin [4] is consistent with the lower prevalence rates we found in studies that detected eggs in stool samples rather than using the scotch tape technique.
- For effective infection control and health promotion in children, it is important to identify the factors that help identify parasite transmission as well as aid in its prevention [9]. Although Enterobius is susceptible to some anthelminthic drugs including mebendazole and albendazole, these drugs only kill the adult worm and are not effective against eggs and larvae. The main point is that medical treatment is not sufficient to cure and control enterobiasis and does not prevent re-infection [13].
- To reduce prevalence rates, effective health promotion includes increasing the awareness of the child and family. Because of the high prevalence of re-infection with enterobiasis, health education and screening programs must be provided for the children, teachers, and parents who gather in crowded places like schools, kindergartens, and daycare centers [9]. One of the most effective control strategies for Enterobius infection is to promote knowledge among families, especially mothers and children, and change their hygiene behaviors [56].
- Various studies have confirmed that increasing knowledge and the hygiene practice levels of families is a suitable control strategy. In Egypt, the Republic of Korea, and Cameroon, decreasing prevalence rates have been reported as a result of health education provided to mothers, the distribution of educational brochures to the children and their families, and visual educational cards, respectively [16,56,58]. As a result of control and screening programs, a decreasing trend in the prevalence rate of E. vermicularis infection has been reported in some countries [9]. In the Republic of Korea and Greece, the prevalence rates dropped from 17.1% to 7.9% and 22.1% to 5.2%, respectively. In Turkey, another study reported the infection rate from 1985 to 2000 was 45.9%, while it was 16% from 2000 to 2008, similar to our findings [16,59–61].
- Our systematic review and meta-analysis had certain limitations: (1) lack of uniform sample size, (2) differing diagnostic methods with varied sensitivity and specificity, and (3) heterogeneity in our review results due to the heterogeneity of different articles. These factors may have biased the prevalence of E. vermicularis infection in the study population.
Discussion
- Although there were many country-specific studies on the prevalence of enterobiasis in children, there were no comprehensive studies describing the status of enterobiasis infection in children globally. Our systematic review and meta-analysis estimated that 12.9% of children around the world are infected with E. vermicularis. Health education for children and their families is a cost-effective and safe control strategy that can decrease the burden of enterobiasis infection. Infection, especially enterobiasis, increases the financial burden of preventative medicine programs, part of which must be paid by the government. Therefore, it is necessary that health policymakers establish effective screening and training programs to eliminate enterobiasis.
Conclusion
HIGHLIGHTS
-
Ethics Approval
This study received approval from the Qazvin University of Medical Sciences Ethical Committee Iran under the contract no. IR.QUMS.REC.1401.291.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
All data generated or analyzed during this study are included in this published article.
-
Authors’ Contributions
Conceptualization: SD, EKL; Data curation: BH, KT; Software: SAH, AJ; Interpretation of data: AM, AT; Writing–original draft: SAH, BH, KT, AJ; Writing–review & editing: AM, SD, EKL, AT. All authors read and approved the final manuscript.
-
Additional Contributions
We are grateful to engineer Mr. Mostafa Sargol for his help.
Article information

| No. | Study | Publication year | Country | Population group | Age group (y) | Sample size (n) | Positive (n) | Diagnostic methods | STROBE score |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Changsap et al. [17] | 2002 | Thailand | Primary school children | 5, 6 | 1,139 | 255 | Cellophane tape swab | 17.5 |
| 2 | Song et al. [18] | 2003 | Republic of Korea | Preschool children | ≤5 | 552 | 35 | Cellophane tape swab | 26 |
| 3 | Nithikathkul et al. [19] | 2003 | Thailand | Children | 1–6 | 42 | 5 | Cellophane tape swab | 20 |
| 4 | Tashima and Simoes [20] | 2004 | Brazil | Children | 1–4 | 420 | 1 | Cellophane tape swab | 18 |
| 5 | Park et al. [8] | 2005 | Republic of Korea | Kindergartens and primary schools | 3–6 | 365 | 70 | Cellophane tape swab | 23 |
| 6 | Tang and Luo [21] | 2005 | China | Children | 2–6 | 1,749 | 136 | Kato-Katz method | 17 |
| 7 | Cazorla et al. [22] | 2006 | Venezuela | Preschool and school children | 2–6 | 241 | 143 | Cellophane tape swab | 26 |
| 8 | de Carvalho et al. [23] | 2006 | Brazil | School children | 0–6 | 279 | 28 | Cellophane tape swab | 25 |
| 9 | Kang et al. [16] | 2006 | Republic of Korea | Preschool children | 0–6 | 1,507 | 119 | Cellophane tape swab | 21 |
| 10 | Remm [24] | 2006 | Estonia | Nursery school children | 1–6 | 372 | 167 | Cellophane tape swab | 29 |
| 11 | Muge et al. [25] | 2008 | Turkey | Preschool children | 5, 6 | 797 | 37 | Cellophane tape anal swab | 19 |
| 12 | Kim et al. [26] | 2010 | Republic of Korea | Kindergartens children | 1–5 | 695 | 96 | Cellophane tape swab | 26 |
| 13 | Goncalves et al. [27] | 2011 | Brazil | Preschool children | 0.5–6 | 133 | 2 | Lutz method | 26 |
| 14 | Hong et al. [28] | 2011 | Republic of Korea | Preschool children | 0–6 | 5,704 | 209 | Cellophane tape swab | 25 |
| 15 | Lee et al. [29] | 2011 | Republic of Korea | Preschool children | 2–6 | 896 | 52 | Cellophane tape swab | 28 |
| 16 | Rashid et al. [30] | 2011 | Bangladesh | Children | 1–6 | 52 | 8 | Cellophane tape swab | 25 |
| 17 | Masucci et al. [31] | 2011 | Italy | Patient population | <5 | 387 | 1 | Formalin-ethyl acetate concentration | 32 |
| 18 | Sayasone et al. [32] | 2011 | Laos | Random population | <5 | 116 | 3 | Formalin-ethyl acetate concentration | 34 |
| 19 | Canete et al. [33] | 2012 | Cuba | Children who attend a day care center | 0.5–5 | 104 | 2 | Cellophane tape swab | 31 |
| 20 | Chu et al. [34] | 2012 | Taiwan | Preschool children | 1–6 | 6,661 | 30 | Cellophane tape swab | 21 |
| 21 | Ebrahimzadeh et al. [35] | 2014 | Iran | Preschool children among kindergartens | 1–6 | 907 | 219 | Cellophane tape swab | 21 |
| 22 | Jiang and Li [36] | 2013 | China | Kindergarten children | 1–6 | 1,088 | 12 | Cellophane tape swab | 20 |
| 23 | Kim et al. [37] | 2013 | Republic of Korea | Kindergarten children | 1–6 | 3,422 | 205 | Cellophane tape swab | 22 |
| 24 | Hegazy et al. [38] | 2014 | Egypt | Preschool children | 2–6 | 500 | 17 | Cellophane tape swab | 25 |
| 25 | Chai et al. [39] | 2015 | Myanmar | Primary school children | 5–6 | 761 | 359 | Cellophane tape swab | 28 |
| 26 | Yentur Doni et al. [40] | 2015 | Turkey | Children | 0–6 | 333 | 126 | Cellophane tape swab | 32 |
| 27 | Li et al. [11] | 2015 | China | Children | 2–6 | 508 | 295 | Cellophane tape swab | 22 |
| 28 | Afrakhteh et al. [41] | 2016 | Iran | Kindergarten and preschool children | 2–6 | 462 | 33 | Cellophane tape swab | 20 |
| 29 | Amiri et al. [42] | 2016 | Iran | Kindergarten children | 2–6 | 126 | 28 | Cellophane tape swab | 25 |
| 30 | Anuar et al. [43] | 2016 | Malaysia | Kindergarten and preschool children | 1–6 | 136 | 17 | Cellophane tape swab | 25 |
| 31 | Haratipour et al. [44] | 2016 | Iran | Kindergarten children | 4–6 | 1,850 | 230 | Cellophane tape and formalin-ether concentration | 23 |
| 32 | Mansourian et al. [45] | 2016 | Iran | Kindergarten children | 2–6 | 175 | 61 | Cellophane tape swab | 24 |
| 33 | Shahdoust et al. [46] | 2016 | Iran | Individuals referred to medical centers | 0–6 | 820 | 4 | Microscopic formalin-ether concentration | 25 |
| 34 | Wang et al. [47] | 2016 | China | Preschool children | 2–6 | 510 | 65 | Cellophane tape swab | 27 |
| 35 | Wang et al. [47] | 2016 | China | Preschool children | 2–6 | 1,734 | 89 | Cellophane tape swab | 27 |
| 36 | Sakari et al. [48] | 2017 | Kenya | Preschool children | 2–5 | 361 | 3 | Kato Katz-direct smears | 33 |
| 37 | Chen et al. [49] | 2018 | Taiwan | Kindergarten and preschool children | 1–6 | 22,776 | 57 | Cellophane tape swab | 24 |
| 38 | Dudlova et al. [50] | 2018 | Slovakia | Preschool children | 3–6 | 159 | 8 | Cellophane tape swab | 24 |
| 39 | Goes et al. [51] | 2019 | Brazil | Preschool children | 1–6 | 121 | 1 | Formalin-ethyl acetate concentration | 25 |
| 40 | Khazaal et al. [52] | 2020 | Iraq | Schools, kindergartens, pediatric hospitals | 2–6 | 216 | 24 | Cellophane tape swab | 22 |
| 41 | Tavan et al. [53] | 2020 | Iran | Kindergarten children | 4–6 | 500 | 15 | Cellophane tape swab | 21 |
| 42 | Tavan et al. [53] | 2020 | Iran | Kindergarten children | 4–6 | 500 | 12 | Cellophane tape swab | 21 |
- 1. Ummarino A, Caputo M, Tucci FA, et al. A PCR-based method for the diagnosis of Enterobius vermicularis in stool samples, specifically designed for clinical application. Front Microbiol 2022;13:1028988. ArticlePubMedPMC
- 2. Dutto M, Montu D, Raineri G. Enterobiasis in pediatric subjects in north-western Italy: a study of home remedies. Ann Ig 2012;24:81−4.PubMed
- 3. World Health Organization (WHO). Deworming for health and development: report of the Third Global Meeting of the Partners for Parasite Control. WHO; 2005.
- 4. Ali WA. Enterobius vermicularis infection: prevalence and risk factors among primary school children in Al-mudhafar Directorate, Taiz, Republic of Yemen. Enhanc Knowl Sci Technol 2022;2:441−9.
- 5. Knudson A, Lemos E, Ariza Y, et al. Frequency of E. Vermicularis in a rural school population of Quipile, Colombia, 2001. Rev Salud Publica (Bogota) 2003;5:87−99.PubMed
- 6. Cazorla DJ, Acosta ME, Zarraga A, et al. Clinical and epidemiological study of enterobiasis in preschool and schoolchildren from Taratara, Falcon State, Venezuela. Parasitol Latinoam 2006;61:43−53. Spanish.
- 7. Boas H, Tapia G, Sodahl JA, et al. Enterobius vermicularis and risk factors in healthy Norwegian children. Pediatr Infect Dis J 2012;31:927−30.ArticlePubMed
- 8. Park JH, Han ET, Kim WH, et al. A survey of Enterobius vermicularis infection among children on western and southern coastal islands of the Republic of Korea. Korean J Parasitol 2005;43:129−34.ArticlePubMedPMC
- 9. Moosazadeh M, Abedi G, Afshari M, et al. Prevalence of Enterobius vermicularis among children in Iran: a systematic review and meta-analysis. Osong Public Health Res Perspect 2017;8:108−15.ArticlePubMedPMC
- 10. Bisetegn H, Debash H, Ebrahim H, et al. Prevalence and determinant factors of intestinal parasitic infections and undernutrition among primary school children in North-Central Ethiopia: a school-based cross-sectional study. J Parasitol Res 2023;2023:2256910. ArticlePubMedPMCPDF
- 11. Li HM, Zhou CH, Li ZS, et al. Risk factors for Enterobius vermicularis infection in children in Gaozhou, Guangdong, China. Infect Dis Poverty 2015;4:28. ArticlePubMedPMCPDF
- 12. Akram HE, Al-Warid HS. Evaluation of hematological factors and micronutrients among children infected with Enterobius vermicularis. Iraqi J Sci 2023;64:1625−34.ArticlePDF
- 13. Ngegba MP, Ngegba AM, Hinckley ES, et al. Implications of prevalence and intensity of soil-transmitted helminthes (STHs) on rural farmers’ productivity in selected districts of Sierra Leone. Zeszyty Naukowe Szkoły Głównej Gospodarstwa Wiejskiego w Warszawie Problemy Rolnictwa Światowego 2023;23:32−45. Polish.ArticlePDF
- 14. Al-Daoody AA, Qadir FM, Tahir AA, et al. Risk factors of Enterobius vermicularis infection with symptoms among children in Erbil Governorate. Pak-Euro J Med Life Sci 2020;3:50−8.
- 15. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell; 2008.
- 16. Kang S, Jeon HK, Eom KS, et al. Egg positive rate of Enterobius vermicularis among preschool children in Cheongju, Chungcheongbuk-do, Korea. Korean J Parasitol 2006;44:247−9.ArticlePubMedPMC
- 17. Changsap B, Nithikathkul C, Boontan P, et al. Enterobiasis in primary schools in Bang Khun Thian District, Bangkok, Thailand. Southeast Asian J Trop Med Public Health 2002;33 Suppl 3:72−5.PubMed
- 18. Song HJ, Cho CH, Kim JS, et al. Prevalence and risk factors for enterobiasis among preschool children in a metropolitan city in Korea. Parasitol Res 2003;91:46−50.ArticlePubMedPDF
- 19. Nithikathkul C, Polseela P, Poodendan W, et al. Malaria and enterobiasis among Karen Long-neck tribe in Mae Hong Son Province. Southeast Asian J Trop Med Public Health 2003;34 Suppl 2:25−8.PubMed
- 20. Tashima NT, Simoes MJ. Enteroparasitic occurrence in fecal samples analyzed at the University of Western São Paulo-UNOESTE Clinical Laboratory, Presidente Prudente, São Paulo State, Brazil. Rev Inst Med Trop Sao Paulo 2004;46:243−8.ArticlePubMed
- 21. Tang N, Luo NJ. Prevalence of parasites in kindergarten children. Int J Infect Dis 2005;9:178−9.ArticlePubMed
- 22. Cazorla D, Acosta M, García E, et al. Enterobius vermicularis infection in preschool and schoolchildren of six rural communities from a semiarid region of Venezuela: A clinical and epidemiological study. Helminthologia 2006;43:81−5.ArticlePDF
- 23. de Carvalho TB, de Carvalho LR, Mascarini LM. Occurrence of enteroparasites in day care centers in Botucatu (São Paulo State, Brazil) with emphasis on Cryptosporidium sp., Giardia duodenalis and Enterobius vermicularis. Rev Inst Med Trop Sao Paulo 2006;48:269−73.ArticlePubMed
- 24. Remm M. Distribution of enterobiasis among nursery school children in SE Estonia and of other helminthiases in Estonia. Parasitol Res 2006;99:729−36.ArticlePubMedPDF
- 25. Muge OA, Baykan Z, Artan C. Enterobiasis among preschool children: a study from Kayseri, Turkey. Jpn J Infect Dis 2008;61:482−3.ArticlePubMed
- 26. Kim DH, Son HM, Kim JY, et al. Parents' knowledge about enterobiasis might be one of the most important risk factors for enterobiasis in children. Korean J Parasitol 2010;48:121−6.ArticlePubMedPMC
- 27. Goncalves AL, Belizario TL, Pimentel Jde B, et al. Prevalence of intestinal parasites in preschool children in the region of Uberlândia, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop 2011;44:191−3.ArticlePubMed
- 28. Hong SH, Lee SE, Jeong YI, et al. Comparison of egg positive rates of Enterobius vermicularis among preschool children in three Korean localities. Korean J Parasitol 2011;49:441−3.ArticlePubMedPMC
- 29. Lee SE, Lee JH, Ju JW, et al. Prevalence of Enterobius vermicularis among preschool children in Gimhae-si, Gyeongsangnam-do, Korea. Korean J Parasitol 2011;49:183−5.ArticlePubMedPMC
- 30. Rashid AK, Rashid AK, Rahman A. Prevalence of intestinal parasitoses in urban and rural children of a developing country. J Trop Biomed 2011;1:S268−70.Article
- 31. Masucci L, Graffeo R, Bani S, et al. Intestinal parasites isolated in a large teaching hospital, Italy, 1 May 2006 to 31 December 2008. Euro Surveill 2011;16:19891. ArticlePubMed
- 32. Sayasone S, Mak TK, Vanmany M, et al. Helminth and intestinal protozoa infections, multiparasitism and risk factors in Champasack province, Lao People's Democratic Republic. PLoS Negl Trop Dis 2011;5:e1037.ArticlePubMedPMC
- 33. Canete R, Diaz MM, Avalos Garcia R, et al. Intestinal parasites in children from a day care centre in Matanzas City, Cuba. PLoS One 2012;7:e51394.ArticlePubMedPMC
- 34. Chu TB, Liao CW, Nara T, et al. Enterobius vermicularis infection is well controlled among preschool children in nurseries of Taipei City, Taiwan. Rev Soc Bras Med Trop 2012;45:646−8.ArticlePubMed
- 35. Ebrahimzadeh A, Saryazdipoor KH, Gharaei AM, et al. Prevalence of Enterobius vermicularis infection among preschool children of Khash city kindergartens, Iran in 2012. J North Khorasan Univ Med Sci 2014;6:477−81.ArticlePDF
- 36. Jiang CG, Li SM. [Investigation of pinworm infection among kindergarten children in Jurong City, Jiangsu Province]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2013;31:355−6. Chinese.PubMed
- 37. Kim DH, Cho MK, Park MK, et al. Environmental factors related to enterobiasis in a southeast region of Korea. Korean J Parasitol 2013;51:139−42.ArticlePubMedPMC
- 38. Hegazy AM, Younis NT, Aminou HA, et al. Prevalence of intestinal parasites and its impact on nutritional status among preschool children living in Damanhur City, El-Behera Governorate, Egypt. J Egypt Soc Parasitol 2014;44:517−24.ArticlePubMed
- 39. Chai JY, Yang SK, Kim JW, et al. High Prevalence of Enterobius vermicularis Infection among Schoolchildren in Three Townships around Yangon, Myanmar. Korean J Parasitol 2015;53:771−5.ArticlePubMedPMC
- 40. Yentur Doni N, Gürses G, Şimşek Z, et al. Prevalence and associated risk factors of intestinal parasites among children of farm workers in the southeastern Anatolian region of Turkey. Ann Agric Environ Med 2015;22:438−42.ArticlePubMed
- 41. Afrakhteh N, Marhaba Z, Mahdavi SA, et al. Prevalence of Enterobius vermicularis amongst kindergartens and preschool children in Mazandaran Province, North of Iran. J Parasit Dis 2016;40:1332−6.ArticlePubMedPDF
- 42. Amiri SA, Rahimi MT, Mahdavi SA, et al. Prevalence of Enterobius vermicularis infection among preschool children, Babol, North of Iran. J Parasit Dis 2016;40:1558−62.ArticlePubMedPMCPDF
- 43. Anuar TS, Jalilah L, Norhayati M, et al. New insights of infection among preschool children in an urban area in Malaysia. Helminthologia 2016;53:76−80.
- 44. Haratipour H, Sohrabi MB, Zolfaghari P, et al. The relationship between malnutrition and intestinal parasitic infections among preschool children in East area of Iran. Int J Pediatr 2016;4:2011−8.
- 45. Mansourian M, Arekhi Z, Jorjani O, et al. Prevalence of Oxyuriasis and its influencing factors in elected kindergartens in Ali Abad-e-Katoul, North of Iran. Int J Pediatr 2016;4:3751−8.
- 46. Shahdoust S, Niyyati M, Haghighi A, et al. Prevalence of intestinal parasites in referred individuals to the medical centers of Tonekabon city, Mazandaran province. Gastroenterol Hepatol Bed Bench 2016;9(Suppl1). S75−9.PubMedPMC
- 47. Wang S, Yao Z, Hou Y, et al. Prevalence of Enterobius vermicularis among preschool children in 2003 and 2013 in Xinxiang city, Henan province, Central China. Parasite 2016;23:30. ArticlePubMedPMC
- 48. Sakari SS, Mbugua AK, Mkoji GM. Prevalence of Soil-Transmitted Helminthiases and Schistosomiasis in Preschool Age Children in Mwea Division, Kirinyaga South District, Kirinyaga County, and Their Potential Effect on Physical Growth. J Trop Med 2017;2017:1013802. ArticlePubMedPMCPDF
- 49. Chen KY, Yen CM, Hwang KP, et al. Enterobius vermicularis infection and its risk factors among pre-school children in Taipei, Taiwan. J Microbiol Immunol Infect 2018;51:559−64.ArticlePubMed
- 50. Dudlova A, Juris P, Jarcuska P, et al. The Incidence of Pinworm (Enterobius Vermicularis) in Pre-school and School Aged Children in the Eastern Slovakia. Helminthologia 2018;55:275−80.ArticlePubMedPMC
- 51. Goes GC, Goncalves KC, Sudre AP, et al. Frequency of enteroparasitoses in preschool children attending daycare centers: a survey applying parasitological and immunological methods. J Trop Pathol 2019;48:121−33.ArticlePDF
- 52. Khazaal R, Al-Hadraawy SK, Hussein KR. Prevalence of Enterobius vermicularis among preschool age and school age children in Thi-Qar province southern Iraq. Int J Pharm Res 2020;857−64.
- 53. Tavan A, Mikaeili F, Sadjjadi SM, et al. Prevalence and genotype distribution of Enterobius vermicularis among kindergarteners in Shiraz and Khorramabad cities, Iran. Asian Pac J Trop Med 2020;13:308−13.Article
- 54. Kim DH, Yu HS. Effect of a one-off educational session about enterobiasis on knowledge, preventative practices, and infection rates among schoolchildren in South Korea. PLoS One 2014;9:e112149.ArticlePubMedPMC
- 55. Pampiglione S, Rivasi F. Enterobiasis in ectopic locations mimicking tumor-like lesions. Int J Microbiol 2009;2009:642481. ArticlePubMedPMCPDF
- 56. Ozdil K, Karatas N, Zincir H. Low socioeconomic level and enterobius vermicularis: a interventional study to children and their mothers in home. Zoonoses Public Health 2020;67:882−91.ArticlePubMedPDF
- 57. Janthu P, Dumidae A, Subkrasae C, et al. Prevalence and genetic analysis of Enterobius vermicularis in schoolchildren in lower northern Thailand. Parasitol Res 2022;121:2955−65.ArticlePubMedPDF
- 58. Mobarak A, Mohamed N, Abd El-Kariem H. Effect of health education program for mothers of children with enterobius vermicularis at Assiut University Children Hospital. Asian Acad Manag J 2011;9:277−306.
- 59. Yang YS, Kim SW, Jung SH, et al. Chemotherapeutic trial to control enterobiasis in schoolchildren. Korean J Parasitol 1997;35:265−9.ArticlePubMed
- 60. Platsouka E, Stephansou T, Marselou-Kinti O. Frequency of Enterobius vermicularis in children from the area of central Greece. Deltion Hellinikis Microbiol Eterial 1985;30:51−9.
- 61. Degerli S, Malatyali E, Ozcelik S, et al. Enterobiosis in Sivas, Turkey from past to present, effects on primary school children and potential risk factors. Turkiye Parazitol Derg 2009;33:95−100.PubMed
References
Figure & Data
References
Citations

- Figure
- Related articles
-
- Predictors of outcomes 3 to 12 months after traumatic brain injury: a systematic review and meta-analysis
- Effects of medication adherence interventions for older adults with chronic illnesses: a systematic review and meta-analysis
- Effect of clofibrate on reducing neonatal jaundice: a systematic review and meta-analysis
- Associations of pre-existing cardiovascular morbidity with severity and the fatality rate in COVID-19 patients: a systematic review and meta-analysis
- Worldwide prevalence of fungal coinfections among COVID-19 patients: a comprehensive systematic review and meta-analysis


 Cite
Cite




