Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Forthcoming articles > Article
-
Special Article
The COVID-19 Vaccine Safety Research Center: a cornerstone for strengthening safety evidence for COVID-19 vaccination in the Republic of Korea -
Na-Young Jeong1,2
 , Hyesook Park1,3,4
, Hyesook Park1,3,4 , Sanghoon Oh1,5
, Sanghoon Oh1,5 , Seung Eun Jung1,4,6
, Seung Eun Jung1,4,6 , Dong-Hyun Kim1,7
, Dong-Hyun Kim1,7 , Hyoung-Shik Shin1,8
, Hyoung-Shik Shin1,8 , Hee Chul Han1,4,9
, Hee Chul Han1,4,9 , Jong-Koo Lee1,4
, Jong-Koo Lee1,4 , Jun Hee Woo1,4
, Jun Hee Woo1,4 , Jaehun Jung1,10,11
, Jaehun Jung1,10,11 , Joongyub Lee1,12
, Joongyub Lee1,12 , Ju-Young Shin1,13,14,15
, Ju-Young Shin1,13,14,15 , Sun-Young Jung1,16,17
, Sun-Young Jung1,16,17 , Byung-Joo Park1,4
, Byung-Joo Park1,4 , Nam-Kyong Choi1,2,18
, Nam-Kyong Choi1,2,18
-
DOI: https://doi.org/10.24171/j.phrp.2023.0343
Published online: April 4, 2024
1COVID-19 Vaccine Safety Research Center, Seoul, Republic of Korea
2Department of Health Convergence, College of Science & Industry Convergence, Ewha Womans University, Seoul, Republic of Korea
3Department of Preventive Medicine, College of Medicine, Graduate Program in System Health Science & Engineering, Ewha Womans University, Seoul, Republic of Korea
4National Academy of Medicine of Korea, Seoul, Republic of Korea
5Department of Psychiatry, Uijeongbu Eulji Medical Center, Eulji University School of Medicine, Uijeongbu, Republic of Korea
6Department of Radiology, Eunpyeong St. Mary’s Hospital, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea
7Department of Social and Preventive Medicine, Hallym University College of Medicine, Chuncheon, Republic of Korea
8Department of Infectious Diseases, Daejeon Eulji Medical Center, Eulji University School of Medicine, Daejeon, Republic of Korea
9Department of Physiology, Korea University College of Medicine, Seoul, Republic of Korea
10Department of Preventive Medicine, Gachon University College of Medicine, Incheon, Republic of Korea
11Artificial Intelligence and Big-Data Convergence Center, Gil Medical Center, Gachon University, Incheon, Republic of Korea
12Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea
13School of Pharmacy, Sungkyunkwan University, Suwon, Republic of Korea
14Department of Biohealth Regulatory Science, Sungkyunkwan University, Suwon, Republic of Korea
15Samsung Advanced Institute for Health Sciences & Technology, Sungkyunkwan University, Seoul, Republic of Korea
16College of Pharmacy, Chung-Ang University, Seoul, Republic of Korea
17Department of Global Innovative Drugs, The Graduate School of Chung-Ang University, Chung-Ang University, Seoul, Republic of Korea
18Graduate School of Industrial Pharmaceutical Science, College of Pharmacy, Ewha Womans University, Seoul, Republic of Korea
- Co-Corresponding author: Byung-Joo Park National Academy of Medicine of Korea, 51 Seochojungang-ro, Seocho-gu, Seoul 06654, Republic of Korea E-mail: bjpark@snu.ac.kr
- Corresponding author: Nam-Kyong Choi Department of Health Convergence, College of Science & Industry Convergence, Ewha Womans University, 52 Ewhayeodae-gil, Seodaemun-gu, Seoul 03760, Republic of Korea E-mail: nchoi@ewha.ac.kr
© 2024 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 478 Views
- 23 Download
Abstract
- The COVID-19 Vaccine Safety Research Committee (CoVaSC) was established in November 2021 to address the growing need for independent, in-depth scientific evidence on adverse events (AEs) following coronavirus disease 2019 (COVID-19) vaccination. This initiative was requested by the Korea Disease Control and Prevention Agency and led by the National Academy of Medicine of Korea. In September 2022, the COVID-19 Vaccine Safety Research Center was established, strengthening CoVaSC’s initiatives. The center has conducted various studies on the safety of COVID-19 vaccines. During CoVaSC’s second research year, from September 29, 2022 to July 19, 2023, the center was restructured into 4 departments: Epidemiological Research, Clinical Research, Communication & Education, and International Cooperation & Policy Research. Its main activities include (1) managing CoVaSC and the COVID-19 Vaccine Safety Research Center, (2) surveying domestic and international trends in AE causality investigation, (3) assessing AEs following COVID-19 vaccination, (4) fostering international collaboration and policy research, and (5) organizing regular fora and training sessions for the public and clinicians. Causality assessments have been conducted for 27 diseases, and independent research has been conducted after organizing ad hoc committees comprising both epidemiologists and clinical experts on each AE of interest. The research process included protocol development, data analysis, interpretation of results, and causality assessment. These research outcomes have been shared transparently with the public and healthcare experts through various fora. The COVID-19 Vaccine Safety Research Center plans to continue strengthening and expanding its research activities to provide reliable, high-quality safety information to the public.
- Since its outbreak in December 2019, coronavirus disease 2019 (COVID-19) rapidly became a global pandemic, with approximately 770 million confirmed cases and 6.9 million fatalities as of October 4, 2023 [1]. To combat the global spread of COVID-19, multiple vaccines have been developed and received emergency use authorization, including vaccines based on an adenovirus vector, protein subunit, and mRNA [2]. In the Republic of Korea, the COVID-19 vaccination program commenced on February 26, 2021, with initial distribution at public health clinics and long-term care centers. As of October 28, 2022, approximately 87.1% of Koreans over 6 months of age had completed their primary vaccine series [3].
- When the COVID-19 vaccination program was initially implemented, the Republic of Korea lacked a robust surveillance system to assess the causal link between COVID-19 vaccines and adverse events (AEs). To strengthen surveillance for scientific and systematic safety assessment, the COVID-19 Vaccine Safety Research Committee (CoVaSC) was established on November 12, 2021 at the request of the Korea Disease Control and Prevention Agency (KDCA) [4]. This initiative was primarily led by the National Academy of Medicine of Korea (NAMOK), an organization comprising experts in medicine, pharmacology, and public health. After its inception, the CoVaSC undertook its first year of research during a 10-month period starting on November 19, 2021. The results of this research were used to expand the list of AEs acknowledged to be causally linked with COVID-19 vaccination [4].
- The first year of research revealed statistically significant risks associated with COVID-19 vaccination and identified additional diseases for inclusion in the list of suspected conditions. Notably, myocarditis and pericarditis were recognized for their causal relationships with BNT162b2 and mRNA-1273 vaccines. Abnormal uterine bleeding related to frequent and irregular menstruation or bleeding was also added to the list of diseases with a suspected association; however, there was insufficient evidence for causality [4].
- During its second year of research, the COVID-19 Vaccine Safety Research Center was established to assume the role of CoVaSC and continue its research mandate. This article describes the research activities of the CoVaSC and the COVID-19 Vaccine Safety Research Center, focusing on COVID-19 Vaccine Safety Assessment. It also outlines the utilization of the findings from the COVID-19 Vaccine Safety Research Center and discusses their implications, providing insights into avenues for future research.
Introduction
- Background for Establishing the COVID-19 Vaccine Safety Research Center
- Following the completion of its first year of research, CoVaSC’s second-year research project began on September 23, 2022 and lasted for 10 months. The COVID-19 Vaccine Safety Research Center was established on September 30, 2022. In November 2022, pursuant to Article 76.2 of the Infectious Disease Control and Prevention Act, the Notice to Designate Agencies for Research and Investigation of COVID-19 Vaccine Safety was enacted. Through this notice, NAMOK was commissioned to conduct safety research and investigate COVID-19 vaccines. This regulatory framework significantly boosted the efficiency of the research process (Table 1).
- Organization of the COVID-19 Vaccine Safety Research Center
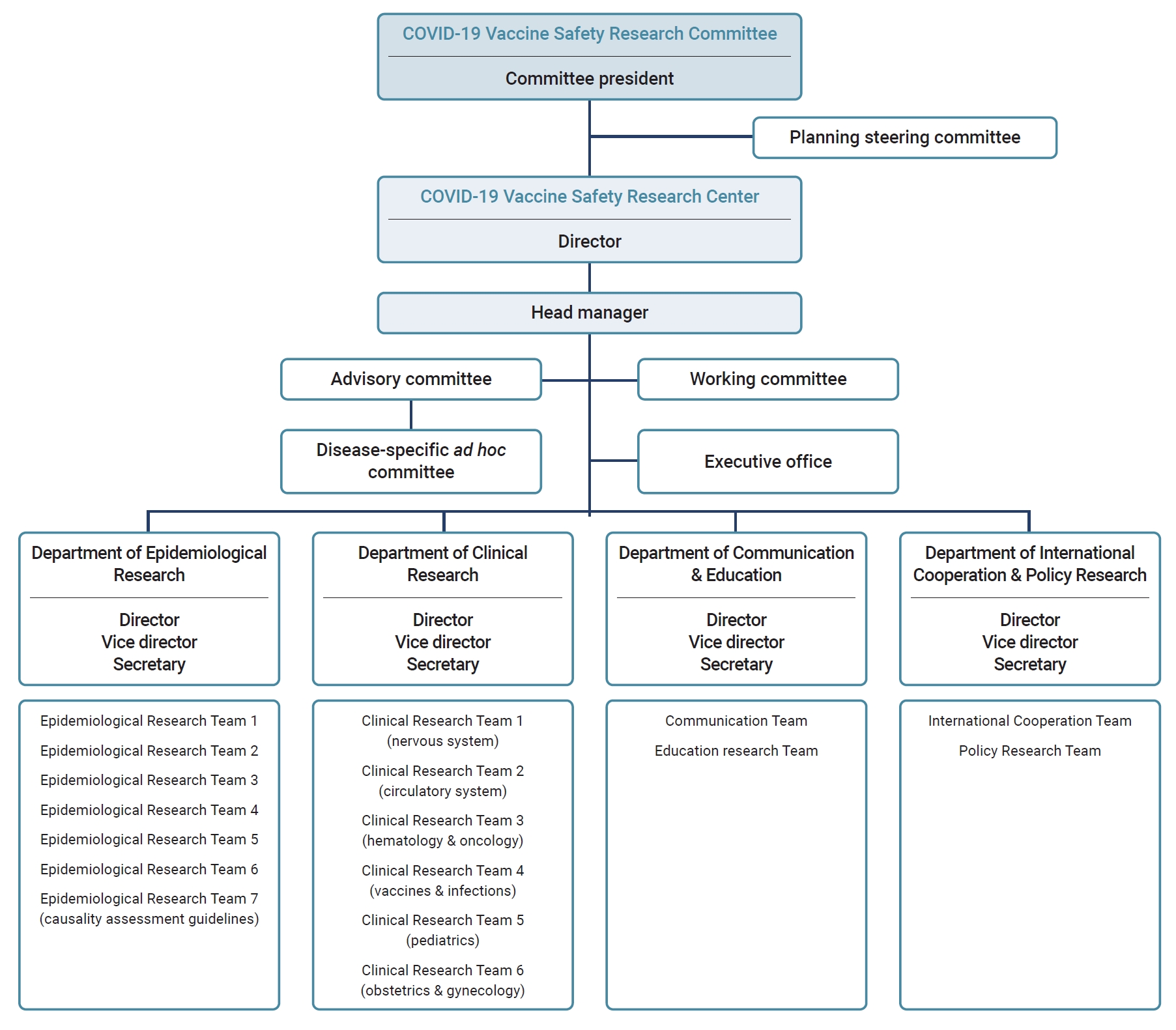
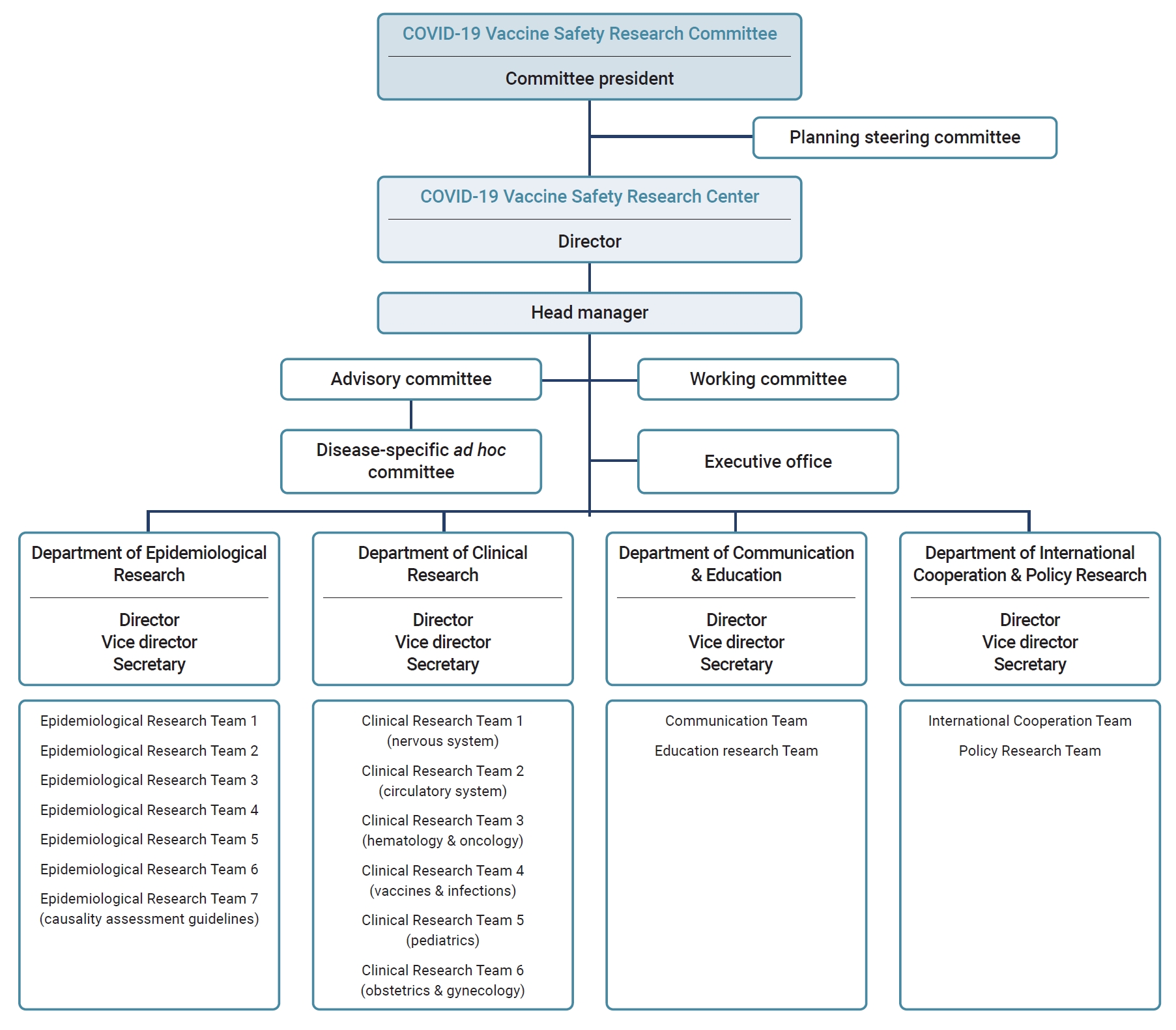
- After the establishment of the COVID-19 Vaccine Safety Research Center under the CoVaSC, it was reorganized, expanding from 3 to 4 departments comprising 17 teams. The Department of Epidemiological Research has 5 teams for research on associations between COVID-19 vaccination and suspected AEs, 1 dedicated to the analysis of AE reports following COVID-19 vaccination, and 1 focused on reviewing causality assessment guidelines. The Department of Clinical Research consists of 6 teams specializing in distinct medical fields. The Department of International Cooperation & Policy Research consists of 2 teams: the International Cooperation Team, which is dedicated to collaborative international studies, and the Policy Research Team, which conducts vaccination-related policy research. The Department of Communication & Education contains teams dedicated to developing communication strategies, public education, and training programs. The center also has an advisory committee consisting of experts in medicine, pharmacology, and public health, along with disease-specific ad hoc committees that collaborate with Epidemiological Research Teams (Figure 1).
- The Department of Epidemiological Research monitors and detects safety signals based on COVID-19 vaccine AE reports, establishes plans for epidemiological research, and performs statistical analyses. The Department of Clinical Research formulates research plans considering the clinical environment and operational definitions. This department conducts preliminary literature reviews on the associations between COVID-19 vaccines, AEs, and biological mechanisms.
- The Department of Clinical Research collaborates with the Department of Epidemiological Research to develop research protocols and to conduct causality assessments based on literature reviews. Ad hoc committees have been organized for each suspected disease of interest by collaboration between the Department of Epidemiological Research and that of Clinical Research, each with clinical experts and epidemiologists related to specific AEs. They have collaboratively developed operational case definitions and interpreted study results to produce high-quality research.
- The Department of International Cooperation & Policy Research releases monthly newsletters to provide updates on international research trends related to COVID-19 vaccine safety. Collaborative research projects have been pursued by agencies responsible for international vaccine safety assessments. The department also organized an international symposium to facilitate collaboration in joint research. The Policy Research Team has explored ways to improve vaccine injury compensation systems and proposed pertinent policy recommendations.
- The Department of Communication & Education develops communication strategies for clinical experts and the public, hosts fora, and issues press releases to share research findings with the COVID-19 Vaccine Safety Research Center. It has also conducted surveys on public perceptions and experiences of COVID-19 vaccination to identify communication needs.
- Scopes and Topics of Research
- The main activities of the COVID-19 Vaccine Safety Research Center include (1) managing CoVaSC and the COVID-19 Vaccine Safety Research Center; (2) surveying domestic and international trends in AE causality investigation; (3) assessing the causality of suspected AEs following COVID-19 vaccination; (4) fostering international collaboration and policy research, and (5) organizing regular fora and training programs aimed at raising awareness and communication with both the public and clinical experts.
- While operating the COVID-19 Vaccine Safety Research Center, the CoVaSC held regular monthly researcher meetings and planning/steering committee meetings to review research progress. Department-specific meetings were arranged separately, and working committee meetings were convened if necessary. Regular and extraordinary meetings were held between the CoVaSC and KDCA to facilitate discussions regarding the diseases to be analyzed and presented, as well as to address general project matters.
- Before commencing its research, the COVID-19 Vaccine Safety Research Center conducted a comprehensive review of the guidelines, protocols, and research findings published by healthcare authorities and vaccine safety surveillance networks in various nations. The center focused on signal detection and causality assessment methodologies and reviewed research methods, previously published articles, and causality assessment algorithms. The COVID-19 Vaccine Safety Research Center selected a causality assessment framework based on an algorithm proposed by the National Academy of Medicine [5] to evaluate the association between COVID-19 vaccines and adverse events of special interest (AESIs).
- To assess the safety of COVID-19 vaccination, the center compiled AESI lists, replicating their approach in the first year. This list was then refined through a review of existing literature and by adopting operational definitions devised primarily by the Department of Clinical Research. The center conducted observed-to-expected analyses to compare the expected incidence of AESIs based on the background rate (AESI occurrence prior to the introduction of COVID-19 vaccines) and the observed incidence after vaccine introduction. Epidemiological studies were carried out using nationwide linked data from the COVID-19 Immunization Registry database at the KDCA and the claims database at the National Health Insurance Service (NHIS), and causality assessments were performed. The center also conducted a descriptive analysis of AE reports from those who completed the primary vaccination series or were vaccinated during the winter. Additionally, the center initiated a survey targeting individuals who reported abnormal uterine bleeding or hair loss as AEs after COVID-19 vaccination. The purpose of this survey was to conduct follow-up studies on symptoms that were difficult to confirm using only linked administrative databases. This survey aimed to gain insights into symptom characteristics, severity, and recovery status.
- To promote international collaboration and policy research, the COVID-19 Vaccine Safety Research Center translated standardized case definitions published by the Brighton Collaboration, a non-profit global vaccine safety research network. The translated versions have been posted on the Brighton Collaboration and COVID-19 Vaccine Safety Research Center websites. Additionally, to promote joint international vaccine safety research, the center signed a Memorandum of Understanding with the Global Vaccine Safety Network on May 19, 2023, to conduct studies based on a common protocol regarding Guillain-Barré syndrome, myocarditis, pericarditis, and vaccine-induced thrombosis and thrombocytopenia. The center organized an international symposium on May 19, 2023, titled “Strengthening International Partnership for Vaccine Safety Research and Beyond.” The conference served as a platform for sharing the research process and discussing opportunities and considerations for future research [6]. Moreover, CoVaSC published a monthly newsletter to provide up-to-date information and knowledge on vaccination status and safety surveillance, both domestically and internationally.
- The Policy Research Team reviewed the compensation programs for post-immunization AEs in the Republic of Korea and abroad to explore ways of improving the existing system. The team examined how vaccine injury compensation programs work globally, paying attention to those that closely resemble the healthcare settings of the Republic of Korea to gain insights into how to improve the current compensation system.
- To improve communication between healthcare professionals and the public, the center conducted expert training sessions on the causality assessment of AEs related to COVID-19 vaccination and guidelines for reporting suspected AEs. Monthly fora were live-streamed on YouTube to disseminate the center’s research findings to the public. These fora shared causality study results and their clinical interpretations, followed by panel discussions with designated panelists. Furthermore, the COVID-19 Vaccine Safety Research Center surveyed the general population and medical professionals to understand their experiences, attitudes, and perceptions regarding COVID-19 vaccination and improve messaging for future vaccinations against new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants.
- Causality Assessment between COVID-19 Vaccination and Suspected AEs
- During the first year of research, 44 AESIs were identified through a literature review and consultation with clinical experts. In the second year, the list of AESIs was refined by removing diseases for which the analysis had concluded and updated to include only conditions requiring further analysis or reevaluation. The prioritization of AESIs included factors such as the incubation period, latent period post-vaccination, potential for relapse, and the feasibility of defining a risk window. The center also assessed whether the AEs had been captured in existing case reports and if pre-existing studies had provided operational definitions of the AEs based on health insurance claims data.
- The final list of AESIs was formulated considering the center’s opinions, the KDCA’s comments, and the need to establish scientific evidence. Table 1 lists the AESIs for which the COVID-19 Vaccine Safety Research Center performed causality assessments during the first and second years of research.
- The COVID-19 Vaccine Safety Research Center utilized a nationwide linked database that combined the NHIS insurance claims database and the KDCA’s COVID-19 Immunization Registry [4], replicating their approach from the first year. The NHIS database included data on all medical services covered by National Health Insurance for the entire Korean population from January 2002 to the most recent data available at the time of analysis, while the KDCA registry provided information on COVID-19 vaccination, AE reports, and details of previously confirmed SARS-CoV-2 cases starting from January 2020. The NHIS linked the COVID-19 vaccine registry with claims data using national registration numbers and provided it in a pseudonymized format to the COVID-19 Vaccine Safety Research Center for analysis.
- For the analysis of AESIs, the expected incidence after COVID-19 vaccination was predicted based on the incidence before the COVID-19 vaccine roll-out. The center conducted an observed-to-expected analysis by calculating the ratio of the observed to expected incidence rates. A seasonal autoregressive integrated moving average model, a well-known time-series forecasting model, was employed.
- For epidemiological association studies on AESIs, the Department of Epidemiological Research and an ad hoc committee adopted appropriate research methodologies, such as self-controlled case series and self-controlled risk intervals. The findings of the literature review and clinical insights from the ad hoc committee were considered to determine the risk window and follow-up period for each disease.
- Following an investigation into the association between COVID-19 vaccination and an AESI, an independent team performed a causality assessment based on the findings of the investigation and a literature review. This team comprised Epidemiological Research Teams, the ad hoc committee from the previous association study, and relevant experts. In the first year of research, the Bradford Hill criteria [7] and the U.S. Surgeon General’s epidemiological criteria for causality [8] were used to determine whether each association was likely to be causal. During the second year of research, the COVID-19 Vaccine Safety Research Center used a framework based on algorithms proposed by the National Academy of Medicine [5] to conduct a population-level causality assessment. In this framework, epidemiological and mechanistic evidence were first evaluated separately and then assessed in a combined manner.
- In performing the causality assessment, the weights of evidence from the epidemiological (including observational studies and randomized controlled trials) and mechanistic literature (including case reports, clinical trials, and clinical and biological studies involving humans, animals, or in vitro models) were assessed separately. Weight-of-evidence assessments for epidemiological evidence were categorized as high, moderate, limited, or insufficient. Mechanistic evidence was classified as strong, intermediate, weak, or lacking. Based on these evaluations, the independent causality assessment committee formulated conclusions, categorizing causality as convincingly supported, favoring acceptance, favoring rejection, inadequate to accept, or inadequate to reject [5].
Materials and Methods
List of AESIs
Data sources
Study design
Causality assessment
- The COVID-19 Vaccine Safety Research Center extensively analyzed the safety of COVID-19 vaccines by monitoring AE reports and examining their potential association with AESIs. The center evaluated the causal relationship between COVID-19 vaccines and AESIs and hosted fora to share its findings. The results of the second year of research were presented at 7 fora held on December 5, 2022, and January 31, February 28, March 30, April 27, May 25, and June 21, 2023.
- The center systematically monitored AE reports following COVID-19 vaccination, encompassing both monovalent and bivalent vaccines, including COVID-19 winter booster vaccines. The analysis explored the demographic characteristics, vaccine types, and dosages among individuals who reported AEs and the factors attributable to AE reporting. It also focused on abnormal uterine bleeding, which exhibited a statistically significant risk, analyzing the details of these reports to identify reporting patterns and calculate reporting rates.
- The key findings from the COVID-19 Vaccine Safety Assessment for the second year are as follows. Eight diseases showed a statistically significant risk with COVID-19 vaccines: acute transverse myelitis, acute disseminated encephalomyelitis, Bell’s palsy/facial nerve disorders, thrombosis with thrombocytopenia syndrome, encephalitis/encephalopathy, herpes zoster, lymphadenitis, and anaphylaxis (Table 2). The results of the studies on each AE were based on announcements at monthly fora and did not undergo formal peer review.
- For 5 diseases, evidence was found favoring the acceptance of a causal relationship or evidence convincingly supporting a causal relationship based on epidemiological and mechanistic evidence: acute transverse myelitis, thrombosis with thrombocytopenia syndrome, encephalitis, encephalopathy, lymphadenitis, and anaphylaxis. For acute disseminated encephalomyelitis, the evidence was deemed inadequate for accepting or rejecting a causal relationship at the population level because few patients were included in the study and associations were absent in the existing mechanistic literature. There is also insufficient mechanistic evidence to conclude that COVID-19 vaccination can cause herpes zoster. Epidemiological studies yielded inconsistent results, making it difficult to accept or reject a causal relationship. For Bell’s palsy and facial nerve disorders, the results do not align with the epidemiological literature, and there is a lack of mechanistic evidence supporting a causal relationship. Therefore, there was inadequate evidence to accept or reject causality. Given the findings from CoVaSC research, the association for acute transverse myelitis, which was initially included in the list of diseases with a suspected association only for adenovirus vector vaccines, expanded to include mRNA vaccines.
- Three diseases showed a low risk after COVID-19 vaccination: Guillain-Barré and Miller-Fisher syndrome, acute respiratory distress syndrome, and interstitial pulmonary diseases. The evidence available to date is inconclusive for accepting or rejecting causal relationships. However, international literature has consistently reported an increased incidence of Guillain-Barré syndrome following the administration of virus vector vaccines. Given its mechanistic possibility, its association with adenovirus vector vaccines was favorable.
Results
- Since their establishment in late 2021, the CoVaSC and COVID-19 Vaccine Safety Research Center have played pivotal roles in investigating the safety of COVID-19 vaccines using domestic data. The studies conducted by CoVaSC have laid the groundwork for a more comprehensive understanding of the side effects associated with these vaccines and expanded the list of diseases recognized to have a causal association with vaccination. Consequently, those affected have been compensated, and new diseases have been listed with their potential associations with the vaccines. Additionally, these studies used domestic data to generate supporting evidence regarding AEs previously recognized for their association with vaccines or included in the list of diseases with a suspected association.
- The research conducted by the CoVaSC and COVID-19 Vaccine Safety Research Center is highly significant. Epidemiological studies using domestic data have produced objective and scientific safety evidence on the association between AEs and COVID-19 vaccination. These findings were disseminated to the public, medical professionals, and governmental bodies. The well-designed research, which was conducted by a wide spectrum of experts (including clinicians specializing in various diseases, epidemiologists, public health experts, and biostatisticians) tapped into the COVID-19 vaccine registry, which included data from the entire population and the health insurance claims database.
- The compiled data serve as a cornerstone for evidence-based policy decisions, particularly regarding compensation and medical support for those who experienced AEs after COVID-19 vaccination. The independence of the research—spearheaded by NAMOK, an academic society rather than a governmental entity—further reinforces public trust. In addition, the CoVaSC conducted international collaborative research and organized global symposia, demonstrating its commitment to global vaccine safety assessments. By sharing its expertise and research results, it collaborated with the international community to respond jointly to the global pandemic.
- There are some limitations to the research carried out by CoVaSC. First, most studies focused on short-term AEs due to the insufficient duration of follow-up for monitoring long-term AEs. Rapid global vaccine roll-out meant that people received vaccinations within the same timeframe. Given the self-controlled study design employed, only abrupt-onset events were defined as outcomes [9]. Second, concerns were raised regarding the validity of the diagnostic codes for certain AESI diagnoses sourced from health insurance claims. It was also difficult to evaluate the causality of diseases that did not require hospital visits. To address this issue, the center devised a plan to build a registry system for conditions with validity concerns (e.g., cerebral venous sinus thrombosis). Third, near-real-time analyses were hampered by the inherent lag time in claims data. To address data delays in future research, we suggest analyzing data on a rolling basis and employing the results for ongoing, routine active surveillance monitoring [10]. Finally, since the research was conducted to inform policymaking using scientific evidence generated from epidemiological studies and population-level causality assessments, the findings may not precisely align with the causality assessment results for individual cases. Furthermore, even with the advantage of a large dataset, the research could not accurately assess extremely rare outcomes [11].
- When the center embarked on its third year of research on July 17, 2023, it was reorganized from 4 to 5 departments. The Department of Causality Assessment was established to strengthen independent causality evaluation. The Department of Communication and Education was renamed the Department of Media Communication with the aim of crafting diverse communication materials to promote collaboration with a wide array of stakeholders for vaccine safety studies. In addition, a geriatrics and immunology team was formed under the Department of Clinical Research.
- Three and a half years after the outbreak of the COVID-19 pandemic, the Korean government lowered the alert level for COVID-19, resulting in the relaxation of several quarantine restrictions. As we transition into the endemic phase, CoVaSC and the COVID-19 Vaccine Safety Research Center are adjusting their research approaches. With routine COVID-19 vaccinations becoming the new norm, the research designs are being redesigned to focus on AEs that may emerge over longer periods. The center also plans to explore as-yet unaddressed issues, including the implications of cumulative vaccine exposure due to multiple booster shots following primary vaccination. In addition, it will strengthen communication efforts with scientists, policymakers, and the public to tackle misunderstandings due to uncertain information and promote open discussions for policymaking to enhance public trust.
Discussion
- Over the past 2 years, the CoVaSC has played a pivotal role in generating scientific evidence to guide policy decisions for potential AEs that may arise from COVID-19 vaccination. Although the global pandemic has subsided and the immediate threat of COVID-19 has diminished, vaccination remains necessary, especially for vulnerable populations. As the COVID-19 Vaccine Safety Research Center has commenced its third-year research project, it will continue to focus on ongoing research and expand its studies to provide reliable safety information to the public.
Conclusion
- • In September 2022, the COVID-19 Vaccine Safety Research Center was established to strengthen the activities of the COVID-19 Vaccine Safety Research Committee (CoVaSC) of the Republic of Korea.
- • The CoVaSC has independently conducted research in collaboration with epidemiologists and clinical experts, ranging from protocol development through data analysis to the interpretation of results. This collaboration has facilitated the causality assessment of 27 adverse events of interest to date.
- • The COVID-19 Vaccine Safety Research Center plans to continue focusing on ongoing research and expanding its studies to provide reliable and high-quality safety information to the public.
HIGHLIGHTS
-
Ethics Approval
This study was approved by the Public Institutional Review Board Designated by the Ministry of Health and Welfare (P01-202203-01-005) and performed in accordance with the principles of the Declaration of Helsinki.
-
Conflicts of Interest
Jong-Koo Lee has been the editor-in-chief of Osong Public Health and Research Perspectives since October 2021, but had no role in the decision to publish this article. No other potential conflict of interest relevant to this article has been declared.
-
Funding
This research was supported by a grant from the Korea Disease Control and Prevention Agency.
-
Authors’ Contributions
Conceptualization: NYJ, JKL, BJP, NKC; Methodology: all authors; Project administration: BJP; Visualization: NYJ; Writing–original draft: NYJ, NKC; Writing–review & editing: all authors. All authors read and approved the final manuscript.
Article information
| Adverse events of interest | Announcement date | Epidemiological study results (statistical significance)a) | Causality assessment results | Policy Measures on the Association Between COVID-19 Vaccines and Adverse Events (vaccine type and time)b) |
|---|---|---|---|---|
| Death | ||||
| All cause death | March 4, 2022 | NS | -d) | |
| Cardio-cerebrovascular disease | ||||
| Myocarditis | March 4, 2022 | Increased (only for mRNA vaccines) | Several criteria are mete) | BNT162b2 and mRNA-127; Mar 2022f) |
| NVX-CoV2373; Dec 2022g) | ||||
| Pericarditis | May 12, 2022c) | Increased (only for mRNA vaccines) | Several criteria are mete) | BNT162b2 and mRNA-1273; May 2022f) |
| NVX-CoV2373; Dec 2022g) | ||||
| Stroke | March 4, 2022 | NS | There are no criteria mete) | |
| Acute myocardial infarction | March 4, 2022 | NS | There are no criteria mete) | |
| Heart failure | May 12, 2022 | NS | Several criteria are mete) | |
| Aortic dissection | May 12, 2022 | NS | Most criteria cannot be evaluatede) | |
| Neurological disease | ||||
| Acute transverse myelitis | January 31, 2023c) | Increased | FA | ChAdOx1 and Ad26·COV2·S; Mar 2022h) |
| BNT162b2 and mRNA-1273; Feb 2023g) | ||||
| Acute disseminated encephalomyelitis | January 31, 2023c) | Increased | I | ChAdOx1h) |
| Guillain-Barré syndrome·Miller-Fisher syndrome | January 31, 2023c) | Decreased | FA (only for adenovirus vector vaccines) or I | ChAdOx1 and Ad26·COV2·Sh) |
| Multiple sclerosis | February 28, 2023 | NS | I | |
| Convulsion/seizures | May 25, 2023 | NS | I | |
| Bell’s palsy/facial nerve disorder | May 25, 2023 | Increased | I | BNT162b2, mRNA-1273, and ChAdOx1h) |
| Encephalitis/encephalopathy | June 21, 2023 | Increased | FA | |
| Encephalomeningitis | June 21, 2023 | NS | I | |
| Obstetric disease | ||||
| Abnormal uterine bleeding | August 11, 2022 | Increased | Several criteria are mete) | All type of vaccines; Aug 2022g) |
| Hematologic disease | ||||
| Deep vein thrombosis | February 28, 2023c) | NS | I | |
| Pulmonary embolism | February 28, 2023c) | NS | I | |
| Cerebral venous sinus thrombosis | August 11, 2022 | Increased | Several criteria are mete) | ChAdOx1 and Ad26·COV2·Sh) |
| Thrombosis with thrombocytopenia syndrome | June 21, 2023c) | Increased | CS | ChAdOx1 and Ad26·COV2·Si) |
| Inflammatory disease | ||||
| Herpes zoster | February 28, 2023 | Increased | I | |
| Lymphadenitis | March 30, 2023 | Increased | CS or FA | All type of vaccinesi) |
| Lupus | May 25, 2023 | NS | I | |
| Respiratory disease | ||||
| Acute respiratory distress syndrome | March 30, 2023 | Decreased | I | |
| Interstitial pulmonary diseases | April 27, 2023 | Decreased | I | |
| Infectious disease | ||||
| Sepsis | April 27, 2023 | NS | FR | |
| Allergic reaction | ||||
| Anaphylaxis | April 27, 2023 | Increased | FA | All type of vaccinesi) |
| Surveillance of adverse event reports | ||||
| Overall adverse event reports | March 4, 2022; May 12, 2022; January 31, 2023; April 27, 2023 | - | -d) | |
| Adverse event reports of abnormal uterine bleeding | March 30, 2023 | - | -d) | |
NS, not significant; FA, favors acceptance of a causal relationship; CS, convincingly supports a causal relationship; FA, favors acceptance of a causal relationship; FR, favors rejection of a causal relationship; I, inadequate to accept or reject a causal relationship.
a) The terms “increased” and “decreased” refer to significantly increased and decreased risk, respectively.
b) This column indicates the timing of policy measures and whether the events were acknowledged as causally related or reportable, following vaccination with each type of COVID-19 vaccine. It also denotes whether the CoVaSC study results had an impact on these policy measures.
c) The outcome was re-analyzed, and only the date to release the results of final re-analysis was included.
d) Causality assessment was not conducted for the adverse event.
e) The Bradford Hill Criteria and the Surgeon General’s epidemiological criteria for causality were used for causal inference.
f) Acknowledgement of causality based on CoVaSC research findings.
g) Added to the list of reportable adverse events based on CoVaSC research findings.
h) Initially listed as reportable adverse events prior to CoVaSC findings.
i) Initially included in the list of adverse events already acknowledged to exhibit a causal relationship before the CoVaSC research.
- 1. World Health Organization (WHO). WHO Coronavirus (COVID-19) dashboard [Internet]. WHO; 2023 [cited 2023 Oct 11]. Available from: https://covid19.who.int/.
- 2. Sandoval C, Guerrero D, Munoz J, et al. Effectiveness of mRNA, protein subunit vaccine and viral vectors vaccines against SARS-CoV-2 in people over 18 years old: a systematic review. Expert Rev Vaccines 2023;22:35−53.ArticlePubMed
- 3. Korea Disease Control and Prevention Agency (KDCA). COVID-19 domestic cases and vaccination status in Republic of Korea [Internet]. KDCA; 2022 [cited 2023 Oct 12]. Available from: https://www.kdca.go.kr/board/board.es?mid=a20501010000&bid=0015&list_no=720988&cg_code=&act=view&nPage=1. Korean.
- 4. Jeong NY, Park H, Oh S, et al. A framework for nationwide COVID-19 vaccine safety research in the Republic of Korea: the COVID-19 Vaccine Safety Research Committee. Osong Public Health Res Perspect 2023;14:5−14.ArticlePubMedPMCPDF
- 5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. The National Academies Press; 2012.
- 6. Jung J. National Academy of Medicine of Korea-Global Vaccine Data Network signs MOU [Internet]. Healthmedia; 2023 [cited 2023 Oct 11]. Available from: http://m.healthmedia.co.kr/news/articleView.html?idxno=88952. Korean.
- 7. Hill AB. The environment and disease: association or causation? Proc R Soc Med 1965;58:295−300.ArticlePubMedPMCPDF
- 8. U.S. Surgeon General's Advisory Committee on Smoking. Smoking and health: report of the Advisory Committee to the Surgeon General of the Public Health Service. US Department of Health, Education, and Welfare, Public Health Service; 1964.
- 9. Gault N, Castaneda-Sanabria J, De Rycke Y, et al. Self-controlled designs in pharmacoepidemiology involving electronic healthcare databases: a systematic review. BMC Med Res Methodol 2017;17:25. ArticlePubMedPMCPDF
- 10. McNeil MM, Gee J, Weintraub ES, et al. The Vaccine Safety Datalink: successes and challenges monitoring vaccine safety. Vaccine 2014;32:5390−8.ArticlePubMedPMC
- 11. World Health Organization (WHO). Causality assessment of an adverse event following immunization (AEFI): user manual for the revised who classification. WHO; 2019.
References
Figure & Data
References
Citations



 Cite
Cite