Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 12(2); 2021 > Article
-
Original Article
COVID-19 transmission: a rapid systematic review of current knowledge -
Panagiotis Mourmouris
 , Lazaros Tzelves
, Lazaros Tzelves , Christiana Roidi
, Christiana Roidi , Anastasia Fotsali
, Anastasia Fotsali
-
Osong Public Health and Research Perspectives 2021;12(2):54-63.
DOI: https://doi.org/10.24171/j.phrp.2021.12.2.02
Published online: April 29, 2021
Second Department of Urology, Athens Medical School, National and Kapodistrian University of Athens, Athens, Greece
- Corresponding author: Panagiotis Mourmouris Second Department of Urology, Athens Medical School, National and Kapodistrian University of Athens, Sismanogleio General Hospital, 1 Sismanogleiou Str, Marousi, Athens, Greece E-mail: thodoros13@yahoo.com
© 2021 Korea Disease Control and Prevention Agency
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- The objective of this study was to identify the potential and definite sources of transmission of coronavirus disease 2019 (COVID-19).
-
Methods
- Due to time constraints and the acute nature of the pandemic, we searched only PubMed/MEDLINE from inception until January 28, 2021. We analyzed the level of evidence and risk of bias in each category and made suggestions accordingly.
-
Results
- The virus was traced from its potential origin via possible ways of transmission to the last host. Symptomatic human-to-human transmission remains the driver of the epidemic, but asymptomatic transmission can potentially contribute in a substantial manner. Feces and fomites have both been found to contain viable virus; even though transmission through these routes has not been documented, their contribution cannot be ruled out. Finally, transmission from pregnant women to their children has been found to be low (up to 3%).
-
Conclusion
- Even though robust outcomes cannot be easily assessed, medical personnel must maintain awareness of the main routes of transmission (via droplets and aerosols from even asymptomatic patients). This is the first attempt to systematically review the existing knowledge to produce a paper with a potentially significant clinical impact.
- Coronavirus disease 2019 (COVID-19) was declared a global pandemic on March 11, 2020 by the World Health Organization; since then, the disease has spread to more than 129 million people and has claimed more than 2,8 milllion lives [1]. This novel coronavirus disease has proven, so far, to be both highly transmittable (unlike severe acute respiratory syndrome [SARS] and Middle East respiratory syndrome [MERS]) and not too fatal (unlike Ebola) [2]. Numerous reports concerning vehicles of transmission have been published, implicating a vast spectrum of possible transmission routes, including fomites [3], asymptomatic transmission (through simple exhalation) [4], and body fluids and secretions. In this systematic review, the first in the current literature according to our knowledge, we analyzed all available data on the possible transmission routes of COVID-19.
Introduction
- Objective
- This study was performed to identify potential and definite sources of transmission of COVID-19.
- Types of Studies
- Due to the acute nature of the pandemic, all types of studies were considered eligible for inclusion; therefore, cohort studies (prospective and retrospective), case reports (including 3 or fewer cases) and case series (more than 3 cases), comments, research letters, laboratory studies, reviews, and meta-analyses were included. We excluded studies that did not focus on transmission routes of the disease. Only studies in English, or studies with an abstract available in English, from which adequate data extraction could be performed, were included.
- Search Methods
- Due to time constraints and the acute nature of the pandemic, we decided to search only PubMed/MEDLINE from inception until January 28, 2021. The snowball procedure was performed in order to identify studies from the references of the included studies. The search was conducted using terms “COVID-19,” “novel coronavirus,” “SARS-CoV-2,” “transmission,” “transmissibility,” and their synonyms using Boolean operators (OR, AND). Two authors (LT, PM) independently searched the database and disagreements were resolved through consensus with a third reviewer.
- Selection of Studies
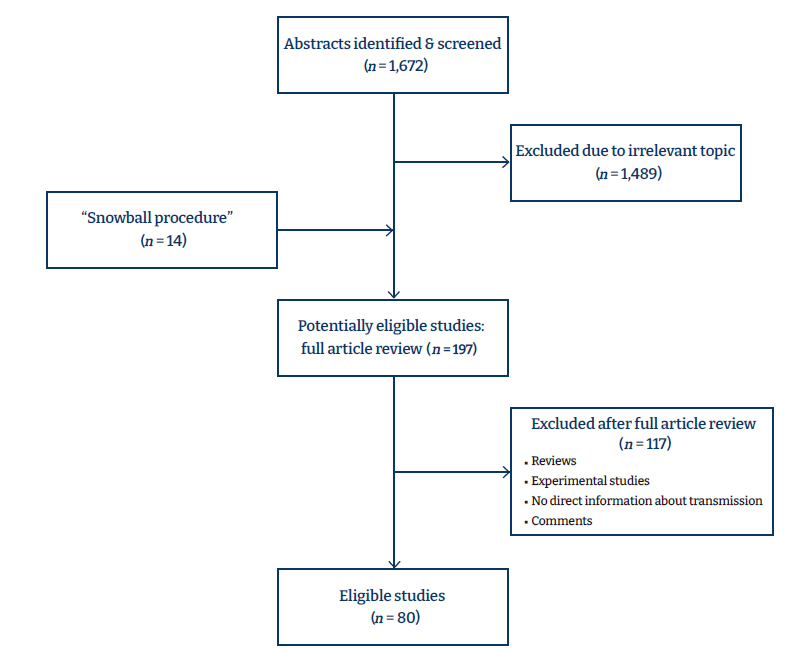
- Two authors (LT, PM) independently assessed the titles and abstracts, and after the initial stage of exclusion of irrelevant studies, they retrieved the full texts for further assessment. A flow diagram (Figure 1) visually depicts the study selection process.
- Data Extraction
- Two authors (LT, PM) independently performed data extraction from the included studies based on a prespecified Excel sheet. The data extracted were relevant to the study characteristics (author, journal, year, country, type), the demographic characteristics of patients (age, sex ratio, proposed route of transmission, comorbidities) and specific data related to the disease (positive samples, duration of positive samples, antibody titers). The last author (AF) reviewed the 2 Excel sheets and disagreements were resolved through consensus.
- Risk of Bias Assessment
- We assessed the risk of bias using the Newcastle-Ottawa scale [5] for cohort studies. Due to the lack of standardized tools for assessing case reports and case series, we used the Joanna Briggs checklist for case series [6] and the criteria established by Pierson [7] to evaluate case reports.
Materials and Methods
- The initial search yielded 1,672 results. After the exclusion of 1,489 studies due to irrelevant topics or a lack of information about the transmission of COVID-19, 197 studies underwent full-text review. The snowball procedure revealed 14 more references, and after the exclusion of 117 additional studies, 80 were finally included in the review (Figure 1). Thirteen were cohort studies [8−20] and the rest were case series, case reports, or studies with other designs. Table 1 presents the characteristics of the studies [3,4,8−62]. To summarize the findings, symptomatic human-to-human transmission remains the main vehicle that drives the epidemic, but asymptomatic transmission can potentially contribute in a substantial manner. Feces and fomites have both been found to contain viable virus; even though transmission through these routes has not been documented, their contribution cannot be ruled out. Finally, the transmission from pregnant women to their children has been found to be low (up to 3%). The risk of bias assessment revealed that 4 cohort studies were of low quality (<6 of 9 stars) according to the Newcastle-Ottawa scale, while most case series and case reports were of good overall quality (Tables S1–S3).
Results
- The most important transmission route of a respiratory virus is the air, but a variety of vehicles are implicated in this mechanism. These vehicles are categorized according to their size: large droplets with a diameter of >20 μm, small particles with a diameter of <5 to 10 μm, and intermediate particles with sizes between 10 and 20 μm [63]. The above-mentioned categorization is of major clinical and epidemiological significance since (a) large droplets cannot follow inhalation streamlines and fall very quickly, following gravity, but can stay on surfaces and produce fomites; and (b) small particles usually evaporate and form residual particulates (aerosols) that can travel in the air, transmit the virus at greater distances, and (if their size is <5 μm) travel deep in the human respiratory tract [64]. Moreover, up-to-date data suggest that exhalations, sneezes, and coughs can produce turbulent gas that not only traps and carries larger droplets, but can significantly decrease their evaporation, thereby extending their lifetime by a factor by up to 1,000 times [65]. The above-mentioned data can—and perhaps should—alter global policies of social distancing and support more aggressive use of face masks among the general population.
- Transmission from Animals to Humans: the Start of the Nightmare
- A laboratory genomic analysis revealed that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, is 96% identical on the whole-genome level to a bat coronavirus CoVZXC21 (RaTG13), and there is a very high similarity (80% sequence identity) between this novel coronavirus and the SARS coronavirus (SARS-CoV-1) that was responsible for the earlier SARS pandemic in the recent past [21]. The study of Xu et al. [22] provides some insights into these issues. The authors found that the human angiotensin converting enzyme 2 (ACE-2) receptor is the gate of viral entry into the human body, while a simple nucleotide replacement (Arg426 with Asn426) increased the binding ability of the novel virus, which may explain its high transmissibility. Although the available data clearly suggest that bats are the reservoir of SARS-CoV-2 and that patient zero was linked to the Wuhan market, the fact that bats are not sold in this market suggests that there may be a possible intermediate host such as snakes, pangolins, or even turtles [66]. The quest to elucidate this major aspect of the pandemic is still ongoing.
- Direct Contact as the Main Vehicle and Airborne Transmission as a Possible
- SARS-CoV-2 is, mainly, a respiratory virus. Therefore, its main route of transmission is contact with droplets produced by a symptomatic patient by coughing, sneezing, or exhaling. We identified 35 studies published in the literature, most of them being case reports or case series, that provide enough evidence for human-to-human transmission through droplets or direct contact with symptomatic patients [3,4,12,13,17,19,23−48,67−70]. All these case series reported either familial clusters, clusters from common indoor places (meetings, buses, temples, hospitals, spas, restaurants), or crowded outdoor facilities (markets). These studies all suggest that transmission of SARS-CoV-2 between humans is relatively easy, as expected. The real problem originates from disturbing data reported in experimental studies suggesting that the virus could remain vital for hours in the air and potentially spread through aerosols. For instance, a study reported that some viable virus particles were present for at least 3 hours in the air and in the form of aerosols [42]. Although conflicting data have been reported on virus transmissibility via aerosols, as well as virus longevity and infectibility in aerosols in real-world settings, these findings are alarming and may have important implications for measures taken by the general public. If this is the case, then a mask should always be used when entering a closed-door room.
- Asymptomatic Transmission
- Unlike SARS-CoV-1 [71], the novel SARS-CoV-2 has an important but likely devastating characteristic: the viral load of asymptomatic or presymptomatic patients is the same as that of symptomatic patients [49,72], although the former may not seem to be as contagious as the latter [15]. It is not known exactly when a presymptomatic patient becomes contagious, but an interval of 2 to 3 days before symptom onset has been suggested [10,26]. Sufficient reports in the literature have suggested asymptomatic transmission from patients who eventually developed symptoms (presymptomatic, with viral shedding during the incubation period) [46] or from patients who were totally asymptomatic, at rates even as high as 50% [4,73], even though most of authors could not definitively prove this assumption [74]. With an estimation that 20% of cases are totally asymptomatic and that the risk ratio of secondary attack from an asymptomatic versus symptomatic patient is 0.35 (95% confidence interval, 0.1−1.27), asymptomatic transmission is an important aspect of the current epidemic [75]. Some research has also reported a link between different mutations of the virus with the infectivity of asymptomatic patients [76].
- Fomites
- It is common knowledge that many contagious diseases can be transmitted through fomites, which are produced by droplets that settle on surfaces after following their trajectory through the air [22]. Some experimental studies have illustrated the presence of the virus on different surfaces after hours or even days in some cases: up to 72 hours for plastic and stainless steel, 8 hours for copper, no more than 24 hours for cardboard [42], and interestingly up to 24 hours on human skin (which makes hand hygiene extremely important) and nearly 8 hours on banknotes [77]. Of course, experimental data provide some insight, but not the same level of evidence as a clinical trial. Guo et al. [78] found that more than 50% of the objects situated in intensive care units and general wards were contaminated by the virus (computer mouse, 75%; bed rails, 43%), whereas the virus was present in other rooms where no patients had been transferred, possibly through staff shoes (half of which were found to be positive). Researchers have found extensive environmental contamination in clinical settings, from patients with only mild upper respiratory tract disease or even without any symptoms (87% of room sites including air outlet fans, table, chairs and bed rails) [3,50]. However, the finding of the utmost clinical significance is the viability of the virus in personal protective equipment, especially due to the global shortage that dictated the need for its reuse. The study by Kasloff et al. [51] provides insights on this important issue: the virus (even at low levels) remained viable for 7 days on nitrile gloves and for almost 21 days in N95 and N100 masks (titers decreased from 24 to 48 hours, stabilized from 48 hours to 4 days and then declined from day 7 to day 21). This clearly demonstrates the need for careful attention in the possible reuse of this equipment to avoid secondary transmission to medical staff. These studies present data from hospital wards and intensive care units (with high loads of virus) and probably do not reflect the transmission dynamics in other settings, and they do not provide any data about possible transmission from surfaces; nevertheless, they demonstrate the relatively high circulation of the virus in our surroundings and therefore the need for surface disinfecting policies [79]. According to a recent systematic review, in household settings, contamination of patients’ surroundings was as high as 14%, with patients’ utensils, electronic high-touch surfaces, beds, and floors representing the most frequently contaminated surfaces [80]. Finally, since fomite transmission is difficult to prove, some publications used mathematical models to demonstrate the contribution of fomites to the growth of the epidemic via transmission, underscoring the need for awareness of this important aspect of transmission [81].
- Fecal Transmission
- ACE-2 receptors are highly expressed in the small intestine [82] and clearly play a role in modulating intestinal inflammation [83]. SARS-CoV-2 utilizes the ACE-2 receptor as the main gate for entering the human body and recent data have suggested that the intestine could serve as a target organ for SARS-CoV-2 [52]. This may explain the gastrointestinal manifestations that are present in a small proportion of patients. There are sufficient data to prove the presence of viral genetic material in patients’ stool [84], making the fecal-oral route a serious candidate for viral shedding. Moreover, viral clearance in stool seems to be even more prolonged than its clearance in nasopharyngeal swabs [14,84], and this fact suggests that patients (especially children) potentially transmit the virus via contaminated feces even after they have been discharged and recovered [48,53,54]. Virus shedding via stool is present even in patients without gastrointestinal symptoms, a fact with significant implications for pandemic control [85]. Despite the above-mentioned data, there are currently no reports of fecal-oral transmission of COVID-19, even though some researchers have reported recovering infectious virus from stool samples [86]. Nevertheless, sharing toilets with an infected person must be discouraged.
- Pregnancy
- The previous pandemic viruses, SARS and MERS, had high fatality rates in pregnant women, which raised theoretical concern regarding their possible risk in the COVID-19 pandemic [87]. We identified 10 studies that dealt with COVID-19 transmission during pregnancy or delivery [8,9,16,18,20,55−58,88]. Vertical transmission (through the placenta) and breastfeeding were investigated. All studies, except for 1, reported negative findings for COVID-19 in neonatal nasopharyngeal swabs, amniotic fluid, and placenta; however, some studies reported positive immunoglobulin M (IgM) and immunoglobulin G antibodies in neonates within hours of birth [18,55], suggesting either a damaged placenta (since IgM antibodies cannot pass through the normal placenta) or virus passage and secondary development of antibodies. It is also important to emphasize that since IgM antibodies develop 3 to 7 days after infection and blood was drawn 2 hours after birth, these antibodies could not have developed due to infection after birth. The infant with a positive throat swab may have acquired the virus due to close contact with the mother, since the authors did not report when the swab was taken and whether the neonate had contact with the mother [58]. Nevertheless, some data have been reported regarding newborns who, despite negative swabs and being separated from the mother without any contact immediately after delivery, developed symptoms suggesting COVID-19 after birth, but the level of evidence is very low for proving vertical transmission [56]. Kotlyar et al. [89] conducted a meta-analysis of 936 SARS-COV-2–tested neonates with COVID-19–positive mothers from 39 studies and identified maternal-to-fetal transmission of the virus in 3.2% of neonates in the third trimester. Regarding breast milk, a recent systematic review found that among 92 newborns whose mothers’ milk was tested, only 4 tested positive for SARS-CoV-2 and 5 were reactive for IgM antibodies. Based on these results and taking into account the benefits of breastfeeding, the Centers for Disease Control and Prevention recommend that women with a suspected or confirmed infection with COVID-19 have no indication to stop breastfeeding [90].
- Other Potential Routes of Transmission
- There are sparse data in the literature about possible transmission routes that cannot contribute to widespread infection, but nevertheless may have implications for everyday life, clinical practice, and the personal protective equipment required for several activities. A confirmed case had positive polymerase chain reaction (PCR) results for SARS-CoV-2 in tears and conjunctival secretions; this patient had conjunctivitis, and the results were negative for patients who did not have conjunctivitis [59]. Xie et al. [60] managed to find traces of SARS-CoV-2 on the ocular surface of patients who did not have any eye symptoms, suggesting that the virus could even spread from the normal conjunctiva, whereas other authors demonstrated that patients with oral swabs negative for SARS-CoV-2 could have positive swabs for the conjunctiva and tears [61]. Similarly, a case report described SARS-CoV-2 isolation in urine samples [62]. The presence of the virus in the blood was proven in 15% of patients in 1 study; although the median PCR cycle value was 35.1, suggesting a very low RNA concentration, this finding suggests the possibility of transmission through blood products [28]. Finally, recent reports reported isolating SARS-CoV-2 in the semen of patients in the acute phase, as well as in 8.7% of patients in the recovery phase [11]. A recent systematic review seems to agree with the above-mentioned results, finding a low but significant rate of patients with SARS-CoV-2–positive semen [91]. Medical personnel and the general population should be aware of these findings to minimize the already low probability of transmission through these routes.
Discussion
Alternative Symptomatic transmission
Supplementary Materials
-
Ethics Approval
Not applicable.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
All data come from the studies included in references.
-
Authors’ Contributions
Conceptualization: PM, LT; Data curation: PM, LT; Formal analysis: LT; Investigation: all authors; Methodology: LT, PM; Project administration: PM; Resources: all authors, Software: all authors; Supervision: PM; Validation: all authors; Visualization: all authors; Writing–original draft: PM; Writing–review & editing: all authors.
Article information
| Study | Type of study | Country | Type of transmission | Vehicle of transmission |
|---|---|---|---|---|
| Xu et al. [22] | Experimental | China | Bat to human | |
| Zhou et al. [21] | Experimental | China | Bat to human | |
| Zhang et al. [48] | Experimental | China, Wuhan | Multiple routes | Droplets, aerosol, feces, blood, serum |
| Dong et al. [55] | Case report | China, Wuhan, Renmin Hospital | Vertical | Amniotic fluid, placenta |
| Yu et al. [58] | Case series | China | Vertical | Amniotic fluid, placenta, close contact |
| Fan et al. [56] | Case report | China | Vertical | Amniotic fluid, placenta, close contact |
| Chen et al. [8] | Retrospective | China, Wuhan Zhongnan Hospital of Wuhan University | Vertical | Amniotic fluid, placenta |
| Li et al. [57] | Case report | China, Zhejiang Province | Vertical | Amniotic fluid, placenta |
| Chen et al. [9] | Retrospective | China, Hubei | Vertical | Amniotic fluid, placenta, close contact |
| Zeng et al. [18] | Retrospective | China, Wuhan Zhongnan Hospital of Wuhan University | Vertical | Amniotic fluid, placenta, close contact |
| Wang et al. [16] | Case report | China, The Affiliated Infectious Hospital of Soochow University | Vertical | Amniotic fluid, placenta |
| Zhu et al. [20] | Retrospective | China, Maternal and Child Health Hospital of Hubei Province | Vertical | Amniotic fluid, placenta |
| Xiao et al. [52] | Case series | China, Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, Guangdong Province | Fecal-oral | Feces |
| Ling et al. [14] | Retrospective | China, Shanghai Public Health Clinical Center | Fecal-oral | Feces |
| Zhang et al. [53] | Case series | China, Tianjiin | Fecal-oral | Feces |
| Xie et al. [60] | Case series | China, Union Hospital, Tongji Medical College, Wuhan | Ocular | Tears |
| Xia et al. [59] | Case series | China, Zhejiang, University School of Medicine First Affiliated Hospital, Hangzhou | Ocular | Tears |
| Sun et al. [62] | Case report | China, Eighth People's Hospital of Guangzhou Medical University | Urine | Urine |
| Cai et al. [23] | Case series | China, Wenzhou | Respiratory contact | Droplets, aerosol, fomites |
| Chan et al. [24] | Case series | China | Respiratory contact | Droplets, aerosol, fomites |
| Ghinai et al. [25] | Case report | USA, Illinois | Respiratory contact | Droplets, aerosol, fomites |
| He et al. [26] | Case series | China, Guangzhou Eighth People’s Hospital | Respiratory contact | Droplets, aerosol, fomites |
| Holshue et al. [27] | Case report | USA, Washington | Unknown | Unknown |
| Huang et al. [28] | Case series | China | Respiratory contact | Droplets, aerosol, fomites |
| Kakimoto et al. [29] | Case series | Japan, Yokohama | Respiratory contact | Droplets, aerosol, fomites |
| Kimball et al. [30] | Case series | USA, Washington | Respiratory contact | Droplets, aerosol, fomites |
| Le et al. [31] | Case report | Vietnam | Respiratory contact | Droplets, aerosol, fomites |
| Li et al. [32] | Case series | China, Zhoushan | Respiratory contact | Droplets, aerosol, fomites |
| Li et al. [12] | Prospective | China, Wuhan | Respiratory contact | Droplets, aerosol, fomites |
| Li et al. [13] | Retrospective | China, Department of Thoracic Surgery Tongji Hospital | Respiratory contact | Droplets, aerosol, fomites |
| Lillie et al. [33] | Case series | UK | Respiratory contact | Droplets, aerosol, fomites |
| Liu et al. [34] | Case report | Taiwan | Respiratory contact | Droplets, aerosol, fomites |
| Luo et al. [35] | Case series | China, Huai’an No. 4 Hospital of Jiangsu Province | Respiratory contact | Droplets, aerosol, fomites |
| Phan et al. [36] | Case report | Vietnam | Respiratory contact | Droplets, aerosol, fomites |
| Pongpirul et al. [37] | Case report | Thailand | Respiratory contact | Droplets, aerosol, fomites |
| Qian et al. [38] | Case series | China, Zhejiang | Respiratory contact | Droplets, aerosol, fomites |
| Rothe et al. [39] | Case series | Germany | Respiratory contact | Droplets, aerosol, fomites |
| Shim et al. [40] | Case series | South Korea | Respiratory contact | Droplets, aerosol, fomites |
| Tong et al. [41] | Case series | China, Zhoushan in Zhejiang Province | Respiratory contact | Droplets, aerosol, fomites |
| van Doremalen et al. [42] | Experimental | USA | Respiratory contact | Droplets, aerosol, fomites |
| Wang et al. [43] | Retrospective | China, Wuhan Zhongnan Hospital of Wuhan University | Respiratory contact | Droplets, aerosol, fomites |
| Wei et al. [17] | Retrospective | China | Respiratory contact | Droplets, aerosol, fomites |
| Xu et al. [44] | Case series | China, Guangzhou Women and Children’s Medical Center | Respiratory contact | Droplets, aerosol, fomites |
| Yu et al. [45] | Case series | China, Wuhan | Respiratory contact | Droplets, aerosol, fomites |
| Yu et al. [46] | Case series | China, Shangai, Wuhan | Respiratory contact | Droplets, aerosol, fomites |
| Zhang et al. [47] | Case series | China | Respiratory contact | Droplets, aerosol, fomites |
| Zhong et al. [19] | Retrospective | China, Zhongnan Hospital, Wuhan | Respiratory contact | Droplets, aerosol, fomites |
| Bai et al. [4] | Case series | China, Anyang | Respiratory contact | Droplets, aerosol, fomites |
| Ong et al. [3] | Case series | Singapore | Respiratory contact | Droplets, aerosol, fomites |
| Li et al. [11] | Prospective | China | Sexual intercourse | Semen |
| Zhao et al. [49] | Case series | China | Respiratory contact | Droplets, aerosol, fomites |
| Sayampanathan et al. [15] | Retrospective | Singapore | Respiratory contact | Droplets, aerosol, fomites |
| Kawasuji et al. [10] | Retrospective | Japan | Respiratory contact | Droplets, aerosol, fomites |
| Lin et al. [54] | Case series | China | Respiratory contact | Droplets, aerosol, fomites |
| Chia et al. [50] | Case series | Singapore | Respiratory contact | Droplets, aerosol, fomites |
| Kasloff et al. [51] | Experimental | Canada | Respiratory contact | Droplets, aerosol, fomites |
| Kaya et al. [61] | Case series | Turkey | Ocular | Tears, conjunctival secretions |
- 1. World Health Organization (WHO). Coronavirus disease 2019 (COVID-19): weekly epidemiological update on Covid 19 [Internet]. Geneva: WHO; 2021 Mar 23 [cited 2021 Mar 30]. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---23-march-2021.
- 2. Yen MY, Schwartz J, Chen SY, et al. Interrupting COVID-19 transmission by implementing enhanced traffic control bundling: implications for global prevention and control efforts. J Microbiol Immunol Infect 2020;53:377−80.ArticlePubMedPMC
- 3. Ong SW, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA 2020;323:1610−2.ArticlePubMedPMC
- 4. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020;323:1406−7.ArticlePubMedPMC
- 5. Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. Ottawa: Ottawa Hospital Research Institute; 2021 [cited 2021 Mar 1]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 6. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk. Edited by Aromataris E, Munn Z: JBI manual for evidence synthesis [Internet]. Adelaide, AU: JBI; 2020. [cited 2021 Mar 30]. Available from: https://doi.org/10.46658/JBIMES-20-08.Article
- 7. Pierson DJ. How to read a case report (or teaching case of the month). Respir Care 2009;54:1372−8.PubMed
- 8. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395:809−15.ArticlePubMedPMC
- 9. Chen S, Liao E, Cao D, et al. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol 2020;92:1556−61.ArticlePubMedPMC
- 10. Kawasuji H, Takegoshi Y, Kaneda M, et al. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS One 2020;15:e0243597.ArticlePubMedPMC
- 11. Li D, Jin M, Bao P, et al. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open 2020;3:e208292.ArticlePubMedPMC
- 12. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199−207.ArticlePubMedPMC
- 13. Li YK, Peng S, Li LQ, et al. Clinical and transmission characteristics of covid-19 - a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci 2020;40:295−300.ArticlePubMedPMC
- 14. Ling Y, Xu SB, Lin YX, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039−43.ArticlePubMedPMC
- 15. Sayampanathan AA, Heng CS, Pin PH, et al. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet 2021;397:93−4.ArticlePubMed
- 16. Wang X, Zhou Z, Zhang J, et al. A case of 2019 novel coronavirus in a pregnant woman with preterm delivery. Clin Infect Dis 2020;71:844−6.ArticlePubMed
- 17. Wei M, Yuan J, Liu Y, et al. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA 2020;323:1313−4.ArticlePubMedPMC
- 18. Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA 2020;323:1848−9.ArticlePubMedPMC
- 19. Zhong Q, Liu YY, Luo Q, et al. Spinal anaesthesia for patients with coronavirus disease 2019 and possible transmission rates in anaesthetists: retrospective, single-centre, observational cohort study. Br J Anaesth 2020;124:670−5.ArticlePubMedPMC
- 20. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr 2020;9:51−60.ArticlePubMedPMC
- 21. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270−3.PubMedPMC
- 22. Xu X, Chen P, Wang J, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci 2020;63:457−60.ArticlePubMedPMC
- 23. Cai J, Sun W, Huang J, et al. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg Infect Dis 2020;26:1343−5.ArticlePubMedPMC
- 24. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514−23.ArticlePubMedPMC
- 25. Ghinai I, McPherson TD, Hunter JC, et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 2020;395:1137−44.PubMedPMC
- 26. He X, Lau EH, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020;26:672−5.ArticlePubMed
- 27. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929−36.ArticlePubMedPMC
- 28. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497−506.ArticlePubMedPMC
- 29. Kakimoto K, Kamiya H, Yamagishi T, et al. Initial investigation of transmission of COVID-19 among crew members during quarantine of a cruise ship - Yokohama, Japan, February 2020. MMWR Morb Mortal Wkly Rep 2020;69:312−3.ArticlePubMedPMC
- 30. Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility: King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020;69:377−81.PubMedPMC
- 31. Le HT, Nguyen LV, Tran DM, et al. The first infant case of COVID-19 acquired from a secondary transmission in Vietnam. Lancet Child Adolesc Health 2020;4:405−6.ArticlePubMedPMC
- 32. Li P, Fu JB, Li KF, et al. Transmission of COVID-19 in the terminal stages of the incubation period: a familial cluster. Int J Infect Dis 2020;96:452−3.ArticlePubMedPMC
- 33. Lillie PJ, Samson A, Li A, et al. Novel coronavirus disease (Covid-19): the first two patients in the UK with person to person transmission. J Infect 2020;80:578−606.Article
- 34. Liu YC, Liao CH, Chang CF, et al. A locally transmitted case of SARS-CoV-2 infection in Taiwan. N Engl J Med 2020;382:1070−2.ArticlePubMedPMC
- 35. Luo C, Yao L, Zhang L, et al. Possible transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in a public bath center in Huai'an, Jiangsu Province, China. JAMA Netw Open 2020;3:e204583.ArticlePubMed
- 36. Phan LT, Nguyen TV, Luong QC, et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N Engl J Med 2020;382:872−4.ArticlePubMedPMC
- 37. Pongpirul WA, Pongpirul K, Ratnarathon AC, et al. Journey of a Thai taxi driver and novel coronavirus. N Engl J Med 2020;382:1067−8.ArticlePubMedPMC
- 38. Qian G, Yang N, Ma AHY, et al. COVID-19 transmission within a family cluster by presymptomatic carriers in China. Clin Infect Dis 2020;71:861−2.ArticlePubMedPMC
- 39. Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020;382:970−1.ArticlePubMedPMC
- 40. Shim E, Tariq A, Choi W, et al. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis 2020;93:339−44.ArticlePubMedPMC
- 41. Tong ZD, Tang A, Li KF, et al. Potential presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg Infect Dis 2020;26:1052−4.ArticlePubMedPMC
- 42. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med 2020;382:1564−7.ArticlePubMed
- 43. Wang X, Pan Z, Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect 2020;105:104−5.ArticlePubMedPMC
- 44. Xu Y, Li X, Zhu B, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med 2020;26:502−5.ArticlePubMedPMC
- 45. Yu J, Ouyang W, Chua ML, et al. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020;6:1108−10.ArticlePubMedPMC
- 46. Yu P, Zhu J, Zhang Z, et al. A familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. J Infect Dis 2020;221:1757−61.ArticlePubMed
- 47. Zhang L, Li J, Zhou M, et al. Summary of 20 tracheal intubation by anesthesiologists for patients with severe COVID-19 pneumonia: retrospective case series. J Anesth 2020;34:599−606.ArticlePubMedPMC
- 48. Zhang W, Du RH, Li B, et al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020;9:386−9.ArticlePubMedPMC
- 49. Zhao H, Lu X, Deng Y, et al. COVID-19: asymptomatic carrier transmission is an underestimated problem. Epidemiol Infect 2020;148:e116.ArticlePubMedPMC
- 50. Chia PY, Coleman KK, Tan YK, et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun 2020;11:2800. ArticlePubMedPMC
- 51. Kasloff SB, Leung A, Strong JE, et al. Stability of SARS-CoV-2 on critical personal protective equipment. Sci Rep 2021;11:984. ArticlePubMedPMC
- 52. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020;158:1831−3.e3.ArticlePubMedPMC
- 53. Zhang T, Cui X, Zhao X, et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol 2020;92:909−14.ArticlePubMedPMC
- 54. Lin GT, Zhang YH, Xiao MF, et al. Epidemiological investigation of a COVID-19 family cluster outbreak transmitted by a 3-month-old infant. Health Inf Sci Syst 2021;9:6. ArticlePubMedPMC
- 55. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020;323:1846−8.ArticlePubMedPMC
- 56. Fan C, Lei D, Fang C, et al. Perinatal transmission of 2019 coronavirus disease-associated severe acute respiratory syndrome coronavirus 2: should we worry? Clin Infect Dis 2021;72:862−4.ArticlePubMed
- 57. Li Y, Zhao R, Zheng S, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2, China. Emerg Infect Dis 2020;26:1335−6.ArticlePubMedPMC
- 58. Yu N, Li W, Kang Q, et al. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis 2020;20:559−64.ArticlePubMedPMC
- 59. Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol 2020;92:589−94.ArticlePubMedPMC
- 60. Xie HT, Jiang SY, Xu KK, et al. SARS-CoV-2 in the ocular surface of COVID-19 patients. Eye Vis (Lond) 2020;7:23. ArticlePubMedPMC
- 61. Kaya H, Çalışkan A, Okul M, et al. Detection of SARS-CoV-2 in the tears and conjunctival secretions of coronavirus disease 2019 patients. J Infect Dev Ctries 2020;14:977−81.ArticlePubMed
- 62. Sun J, Zhu A, Li H, et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerg Microbes Infect 2020;9:991−3.ArticlePubMedPMC
- 63. Cole EC, Cook CE. Characterization of infectious aerosols in health care facilities: an aid to effective engineering controls and preventive strategies. Am J Infect Control 1998;26:453−64.ArticlePubMedPMC
- 64. Tellier R, Li Y, Cowling BJ, et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis 2019;19:101. ArticlePubMedPMC
- 65. Bourouiba L. Turbulent gas clouds and respiratory pathogen emissions: potential implications for reducing transmission of COVID-19. JAMA 2020;323:1837−8.PubMed
- 66. Guo YR, Cao QD, Hong ZS, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res 2020;7:11. ArticlePubMedPMC
- 67. Lai TH, Tang EW, Chau SK, et al. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: an experience from Hong Kong. Graefes Arch Clin Exp Ophthalmol 2020;258:1049−55.ArticlePubMedPMC
- 68. Liu X, Zhang S. COVID-19: face masks and human-to-human transmission. Influenza Other Respir Viruses 2020;14:472−3.ArticlePubMedPMC
- 69. Liu Y, Eggo RM, Kucharski AJ. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet 2020;395:e47.ArticlePubMedPMC
- 70. Nishiura H, Linton NM, Akhmetzhanov AR. Initial cluster of novel coronavirus (2019-nCoV) infections in Wuhan, China is consistent with substantial human-to-human transmission. J Clin Med 2020;9:488. ArticlePubMedPMC
- 71. Peiris JS, Chu CM, Cheng VC, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767−72.ArticlePubMedPMC
- 72. Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020;382:1177−9.ArticlePubMedPMC
- 73. Johansson MA, Quandelacy TM, Kada S, et al. SARS-CoV-2 transmission from people without COVID-19 symptoms. JAMA Netw Open 2021;4:e2035057.ArticlePubMedPMC
- 74. Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the Achilles' heel of current strategies to control Covid-19. N Engl J Med 2020;382:2158−60.ArticlePubMed
- 75. Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLoS Med 2020;17:e1003346.ArticlePubMedPMC
- 76. Wang R, Chen J, Hozumi Y, et al. Decoding asymptomatic COVID-19 infection and transmission. J Phys Chem Lett 2020;11:10007−15.ArticlePubMedPMC
- 77. Harbourt DE, Haddow AD, Piper AE, et al. Modeling the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on skin, currency, and clothing. PLoS Negl Trop Dis 2020;14:e0008831.ArticlePubMedPMC
- 78. Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis 2020;26:1583−91.ArticlePubMed
- 79. Wong JCC, Hapuarachchi HC, Arivalan S, et al. Environmental contamination of SARS-CoV-2 in a non-healthcare setting. Int J Environ Res Public Health 2020;18:117. ArticlePubMedPMC
- 80. Bedrosian N, Mitchell E, Rohm E, et al. A systematic review of surface contamination, stability, and disinfection data on SARS-CoV-2 (through July 10, 2020). Environ Sci Technol 2021;55:4162−73.ArticlePubMed
- 81. Meiksin A. Dynamics of COVID-19 transmission including indirect transmission mechanisms: a mathematical analysis. Epidemiol Infect 2020;148:e257.ArticlePubMedPMC
- 82. Liang W, Feng Z, Rao S, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut 2020;69:1141−3.ArticlePubMed
- 83. Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012;487:477−81.ArticlePubMedPMC
- 84. Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 2020;159:81−95.ArticlePubMedPMC
- 85. Park SK, Lee CW, Park DI, et al. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin Gastroenterol Hepatol 2020;Jun 10 [Epub]. https://doi.org/10.1016/j.cgh.2020.06.005.Article
- 86. Jones DL, Baluja MQ, Graham DW, et al. Shedding of SARS-CoV-2 in feces and urine and its potential role in person-to-person transmission and the environment-based spread of COVID-19. Sci Total Environ 2020;749:141364. ArticlePubMedPMC
- 87. Qiao J. What are the risks of COVID-19 infection in pregnant women? Lancet 2020;395:760−2.ArticlePubMedPMC
- 88. Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med 2020;144:799−805.ArticlePubMed
- 89. Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol 2021;224:35−53.e3.ArticlePubMed
- 90. Rodrigues C, Baía I, Domingues R, et al. Pregnancy and breastfeeding during COVID-19 pandemic: a systematic review of published pregnancy cases. Front Public Health 2020;8:558144. ArticlePubMedPMC
- 91. Gonzalez DC, Khodamoradi K, Pai R, et al. A systematic review on the investigation of SARS-CoV-2 in semen. Res Rep Urol 2020;12:615−21.PubMedPMC
References
Figure & Data
References
Citations

- Should We Interfere with the Interleukin-6 Receptor During COVID-19: What Do We Know So Far?
Alexia Plocque, Christie Mitri, Charlène Lefèvre, Olivier Tabary, Lhousseine Touqui, Francois Philippart
Drugs.2023; 83(1): 1. CrossRef - Evaluation the efficacy and safety of N‐acetylcysteine inhalation spray in controlling the symptoms of patients with COVID‐19: An open‐label randomized controlled clinical trial
Yunes Panahi, Mostafa Ghanei, Morteza Rahimi, Abbas Samim, Amir Vahedian‐Azimi, Stephen L. Atkin, Amirhossein Sahebkar
Journal of Medical Virology.2023;[Epub] CrossRef - A narrative review of alternative transmission routes of COVID 19: what we know so far
Alyexandra Arienzo, Valentina Gallo, Federica Tomassetti, Nicoletta Pitaro, Michele Pitaro, Giovanni Antonini
Pathogens and Global Health.2023; 117(8): 681. CrossRef - Clinical Application of In Vitro Tests for COVID-19 Vaccine Delayed Hypersensitivity Diagnostics
Jan Romantowski, Aleksandra Górska, Maciej Zieliński, Piotr Trzonkowski, Karolina Rucka, Marek Niedoszytko
International Journal of Molecular Sciences.2023; 24(17): 13296. CrossRef - Bayesian network-based spatial predictive modelling reveals COVID-19 transmission dynamics in Eswatini
Wisdom M. D. Dlamini, Sabelo P. Simelane, Nhlanhla M. Nhlabatsi
Spatial Information Research.2022; 30(1): 183. CrossRef - Fetal inflammatory response syndrome and postnatal multi-system inflammatory syndrome in COVID-19-positive neonates
Meenakshi S. KUSHWAH, Arunkrishnan BALARAVI, Lakshmi VENUGOPALAN, Sreekanth RAMASHENOY, Anita CHRISBINA, Monisha PRABHAKARN, Sumaiya ALAUDDIN, Munmun SAHNEY, Manoj K. DEENADAYALAN, Prakash PETCHIMUTHU
Minerva Respiratory Medicine.2022;[Epub] CrossRef


 PubReader
PubReader ePub Link
ePub Link Cite
Cite

