Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 13(2); 2022 > Article
-
Original Article
Sex differences in factors associated with prediabetes in Korean adults -
Jin Suk Ra

-
Osong Public Health and Research Perspectives 2022;13(2):142-152.
DOI: https://doi.org/10.24171/j.phrp.2022.0053
Published online: April 22, 2022
College of Nursing, Chungnam National University, Daejeon, Korea
- Corresponding author: Jin Suk Ra College of Nursing, Chungnam National University, 266 Munhwa-ro, Jung-gu, Daejeon 35015, Korea E-mail: jinsukra@cnu.ac.kr
© 2022 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- Identifying the factors associated with prediabetes is necessary for the early detection and management of high-risk individuals with prediabetes. The purpose of this study was to identify the factors associated with prediabetes according to sex in Korean adults.
-
Methods
- Using the Korean National Health and Nutrition Examination Survey from 2015 to 2019, a total of 13,595 adults (5,565 males and 8,030 females) aged ≥20 years were included in the data analysis. Logistic regression analysis was performed to identify the factors associated with prediabetes according to sex in Korean adults.
-
Results
- In both males and females, age and a family history of type 2 diabetes were associated with prediabetes. In males, current and past smoking habits were associated with increased prediabetes. In addition, low-intensity physical activity and prolonged sedentary behavior were associated with a higher prevalence of prediabetes. Females with a lower education level (less than middle school graduation) showed a higher risk of prediabetes.
-
Conclusion
- Sex-specific prevention strategies for prediabetes should be developed. In addition, older individuals and those with a family history of type 2 diabetes should be screened for prediabetes.
- Prediabetes is an intermediate metabolic state that is characterized by increased blood glucose levels when compared to normal levels, although not as high as the diagnostic cut-off for diabetes [1,2]. Because individuals with prediabetes do not experience distinctive signs and symptoms, they can be at high risk of developing type 2 diabetes and cardiovascular disease (CVD) [3,4]. According to the National Diabetes Statistics report in 2020, 34.5% of adults over 18 years of age in the United States had prediabetes [5]. In Korea, the prevalence of prediabetes in adults over 30 years was 26.9% in 2018, and the prevalence has continued to increase [6]. Notably, 14.2% to 24.6% of cases of prediabetes in adults over 45 years of age progressed to type 2 diabetes within 10 years [7] and 32.2% of individuals with a diagnosis of type 2 diabetes had CVD approximately 10 years after being diagnosed [8]. Thus, the long-term healthcare burden of unmanaged prediabetes causes substantial public health problems. In this context, we emphasize the need for early identification of at-risk individuals and the early management of prediabetes before the development of type 2 diabetes [9,10]. Identification of the factors associated with prediabetes is required for early intervention and detection of high-risk individuals with prediabetes [11]. Sociodemographic factors (e.g., age, sex, and education level), cardiometabolic factors (e.g., dyslipidemia and hypertension), and behavioral factors (e.g., smoking habits and physical activity) associated with prediabetes have been identified in previous studies [11−14]. However, the reported risk factors for prediabetes have been inconsistent among studies [14,15]. Differences in ethnicity and sex might have contributed to these disparities among studies, in addition to differing definitions of prediabetes [11,16].
- In previous studies, the prevalence of prediabetes and the factors associated with prediabetes differed according to ethnicity [10,17]. Furthermore, in individual ethnic groups, factors associated with prediabetes varied according to sex [10,11,16]. According to a systematic review by Siddiqui et al. [14], smoking and alcohol consumption were significantly associated with prediabetes in males, while poor dietary habits were strongly associated with prediabetes in females. That study emphasized the identification of sex differences in factors associated with prediabetes, which would be helpful for developing sex-specific prevention strategies for those at risk of type 2 diabetes [14].
- According to a conceptual model that explains the development of risk factors and health status related to CVD [18], risk factors and health status (e.g., obesity, type 2 diabetes, and dyslipidemia) related to CVD were associated with sociodemographic determinants (e.g., age, sex, education level, household socioeconomic status), internal health resources/burdens (e.g., psychosocial discomfort), external health resources/burdens (e.g., social support and familial status), and health behaviors (e.g., diet, smoking habit, physical activity). Based on a literature review, this study categorized the factors associated with prediabetes into sociodemographic factors (age, sex, education level, and household socioeconomic status), internal health resources/burdens (stress and depression), external health resources/burdens (living with a spouse, and a family history of type 2 diabetes), and health behaviors (habit of eating out, current and past smoking, current alcohol consumption, physical activity, and sedentary behavior). Adjustments were also made for CVD risk factors (covariates) that are significantly associated with prediabetes, including adiposity (obesity), abdominal obesity, hypertension, high triglyceride (TG) levels, low levels of high-density lipoprotein cholesterol (HDL-C), and high total cholesterol levels.
- This study aimed to identify the associations of sociodemographic, internal health resources/burdens, external health resources/burdens, and health behavioral factors with prediabetes after controlling for covariates according to sex in Korean adults.
Introduction
- Study Design and Population
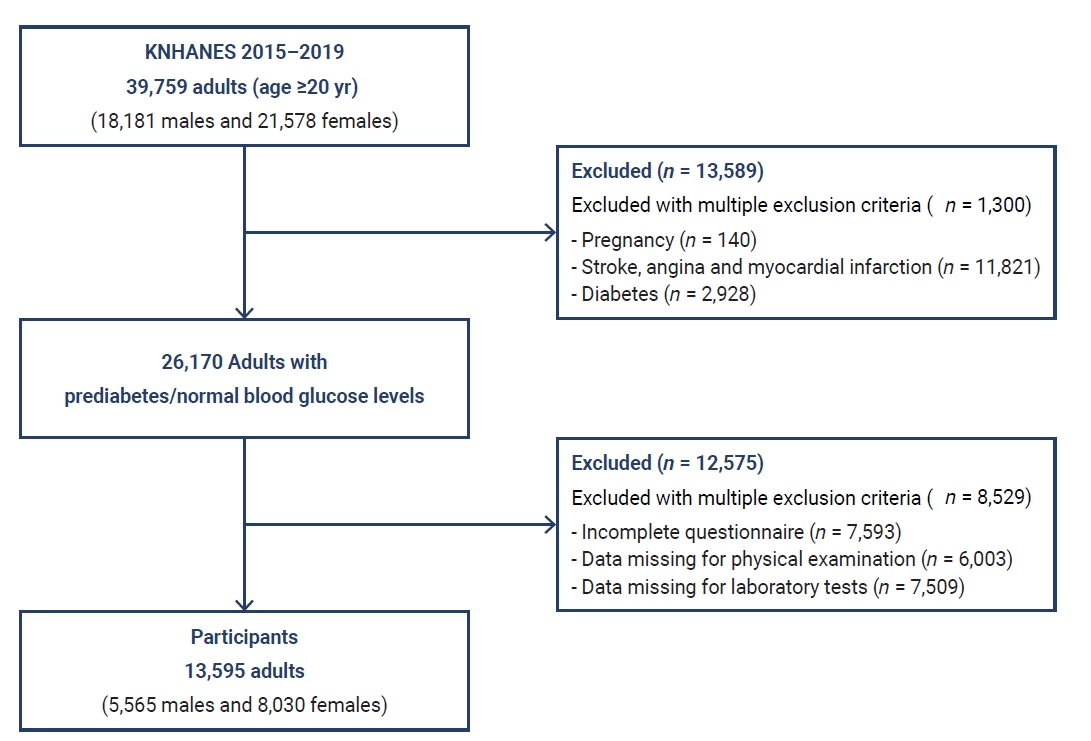
- A cross-sectional study design was applied with a secondary analysis of the Korean National Health and Nutrition Examination Survey (KNHANES) from 2015 to 2019. A total of 39,759 adults aged ≥20 years (18,181 males and 21,578 females) participated in the KNHANES. Among them, 26,170 adults who had prediabetes (11,838 individuals) and normal blood glucose levels (15,332 individuals) were primarily selected after excluding 13,589 adults, including pregnant females and individuals who were diagnosed and treated for stroke, angina, myocardial infarction, and diabetes. Finally, 13,595 adults (5,565 males, 8,030 females) aged ≥20 years were included in the data analysis after excluding 13,575 individuals who had incomplete questionnaires, physical examinations (e.g., weight, height), and laboratory tests (e.g., fasting plasma glucose [FPG] and hemoglobin A1c [HbA1c]) that provided the measurements for the variables used in this study (Figure 1).
- Measurements
- Following the Korean Diabetes Association diagnostic criteria [6,19], the definition of prediabetes was an FPG of 100 to 125 mg/dL and/or an HbA1c of 5.7% to 6.4%. In addition, a normal blood glucose level was defined as an FPG of <100 mg/dL and/or an HbA1c of <5.7%.
- Age was categorized into 20s to 30s, 40s to 50s, and ≥60s. Sex was categorized as male or female. Education level was categorized as less than middle school graduation, high school graduation, or college graduation. Household socioeconomic status was categorized as low, middle, and high.
- Daily stress was determined with a single question, with response categories of “severe,” “a little,” and “not at all.” To evaluate depression, respondents were asked if they had been diagnosed with depression by a psychiatrist, with a response of yes or no.
- Marital status was categorized as living with a spouse or living without a spouse (separated, divorced, bereaved, and unmarried). The family history of type 2 diabetes was categorized as yes or no.
- To evaluate the habit of eating out, the average frequency of eating out in the previous year was determined with a single question, and the response was categorized as “more than once a day” or “less than once a day.” Smoking habits were categorized as current, past, or never, and current alcohol consumption was categorized as “yes” or “no.” For the intensity of physical activity (work, travel to and from places, and leisure), metabolic equivalent task (MET)‐minutes were calculated from activities in a week [20]; >600 MET‐minutes a week was considered moderate to vigorous intensity and <600 MET‐minutes a week was considered low intensity. To evaluate sedentary behavior, the number of hours spent sitting or lying down in a day was determined with a single question, and sedentary hours a day were categorized as <8 hours a day or ≥8 hours a day, with the latter being considered prolonged sedentary behavior [21].
- To evaluate adiposity, body mass index (BMI) was calculated using height and weight. Based on BMI, participants were categorized as non-obese or obese. The non-obese category included underweight (<18.5 kg/m2) and normal weight (≥18.5 kg/m2 and <23 kg/m2), and the obese category included overweight (≥23 kg/m2 and <25 kg/m2) and obesity (>25 kg/m2) [22]. Abdominal obesity was assessed by waist circumference: a waist circumference >90 cm in males and >85 cm in females indicated abdominal obesity [22]. Hypertension was regarded as a blood pressure >130/85 mmHg and/or use of antihypertensive therapy. Other covariates included high total cholesterol, high TG (TG >150 mg/dL and/or treatment for hypertriglyceridemia), and low HDL-C (HDL-C <50 mg/dL and/or therapy for low HDL-C).
- Statistical Analysis
- Complex sampling analysis was performed with IBM SPSS ver. 26.0 (IBM Corp., Armonk, NY, USA) following the data analysis guidelines recommended by the KNHANES. The characteristics of prediabetes, sociodemographic factors, internal and external health resources/burdens, health behavior, and covariates were analyzed using frequencies and percentages. As the first step in logistic regression analysis, univariate logistic regression was conducted to identify the association between each independent and outcome variable. Controlling for covariates, multivariate logistic regression analysis was then performed to identify the factors associated with prediabetes according to sex in Korean adults.
- IRB/IACUC Approval
- This study was approved by the Institutional Review Board of Chungnam National University and was exempted from review because it was a secondary analysis (202111-SB-242-01).
Materials and Methods
Dependent variable
Prediabetes
Independent variables
Sociodemographic factors
Internal health resources/burdens
External health resources/burdens
Health behaviors
Covariates
- The prevalence of prediabetes was 40.9% in males and 34.4% in females (Table 1). The sociodemographic, internal health resources/burdens, external health resources/burdens, and health behavioral characteristics of the males and females are presented in Table 1.
- The factors associated with prediabetes in males are presented in Table 2. In the univariate logistic model for prediabetes in males, age and education level were the sociodemographic factors associated with the prevalence of prediabetes. The following age groups were associated with a lower likelihood of developing prediabetes in males: 20s to 30s (crude odds ratio [COR], 0.17; 95% confidence interval [CI], 0.15–0.19; p<0.001) and 40s to 50s (COR, 0.61; 95% CI, 0.54–0.68; p<0.001). The following factors were associated with a higher risk of developing prediabetes in males: (1) lower education level (less than middle school graduation) (COR, 1.26; 95% CI, 1.14–1.40; p<0.001); (2) among internal health resources/burdens, severe stress (COR, 1.28; 95% CI, 1.14–1.47; p<0.001) or “a little” stress (COR, 1.22; 95% CI, 1.10–1.37; p<0.001); (3) among external resources/burdens, a positive family history of type 2 diabetes (COR, 1.38; 95% CI, 1.27–1.51; p<0.001); and (4) among health behaviors, eating out more than once a day (COR, 1.28; 95% CI, 1.16–1.39; p<0.001), current smoking (COR, 1.98; 95% CI, 1.78–2.19; p<0.001), previous smoking (COR, 2.63; 95% CI, 2.38–2.90; p<0.001), low-intensity physical activity (COR, 1.49; 95% CI, 1.32–1.67; p<0.001), and prolonged sedentary behavior for ≥8 hours a day (COR, 1.39; 95% CI, 1.28–1.52; p<0.001).
- In the multivariate logistic model for prediabetes in males, age was associated with the prevalence of prediabetes. Participants in their 20s to 30s (adjusted odds ratio [AOR], 0.28; 95% CI, 0.22–0.36; p<0.001) and 40s to 50s (AOR, 0.64; 95% CI, 0.51–0.79; p<0.001) had a lower likelihood of developing prediabetes. Among external health resources/burdens, a family history of type 2 diabetes was also associated with a higher likelihood of developing prediabetes (AOR, 1.17; 95% CI, 1.01–1.35, p=0.037). Among health behaviors, current smoking (AOR, 1.35; 95% CI, 1.13–1.63, p=0.001) and past smoking (AOR, 1.29; 95% CI, 1.09–1.53, p=0.004) were associated with a higher likelihood of developing prediabetes. Low-intensity physical activity (AOR, 1.37; 95% CI, 1.19–1.59; p<0.001) and prolonged sedentary behavior for ≥8 hours a day (AOR, 1.22; 95% CI, 1.06–1.41, p=0.006) were associated with a higher likelihood of developing prediabetes in males. The logistic regression model was found to fit the study variables (F=33.29, p<0.001).
- The factors associated with prediabetes in females are presented in Table 3. In the univariate logistic model for prediabetes in females, the sociodemographic factors of age and education level were associated with the prevalence of prediabetes. Younger age groups were associated with a lower likelihood of developing prediabetes: 20s to 30s (COR, 0.09; 95% CI, 0.08–0.10; p<0.001) and 40s to 50s (COR, 0.34; 95% CI, 0.31–0.37; p<0.001). The following factors were associated with a higher likelihood of developing prediabetes in females: (1) lower education level (less than middle school graduation) (COR, 2.91; 95% CI, 2.64–3.20; p<0.001); (2) among internal health resources/burdens, severe stress (COR, 1.89; 95% CI, 1.67–2.13; p<0.001), “a little” stress (COR, 1.61; 95% CI, 1.45–1.79; p<0.001), and depression (COR, 1.46; 95% CI, 1.24–1.72; p<0.001); (3) among external health resources/burdens, a family history of type 2 diabetes (COR, 1.41; 95% CI, 1.31–1.53; p<0.001); and (4) among health behaviors, eating out more than once a day (COR, 1.92; 95% CI, 1.72–2.17; p<0.001), a history of smoking (COR, 1.52; 95% CI, 1.27–1.82; p<0.001), current alcohol consumption (COR, 1.61; 95% CI, 1.47–1.75; p<0.001), low-intensity physical activity (COR, 1.27; 95% CI, 1.14–1.39; p<0.001), and prolonged sedentary behavior for ≥8 hours a day (COR, 1.36; 95% CI, 1.26–1.46; p<0.001).
- In the multivariate logistic model for prediabetes in females, lower education level (less than middle school graduation) showed an association with a higher likelihood of developing prediabetes (AOR, 1.21; 95% CI, 1.04–1.41, p=0.014). The logistic regression model was found to fit the study variables (F=46.28, p<0.001).
Results
- This study identified the factors associated with prediabetes according to sex in Korean adults and showed that the prevalence of prediabetes was higher in Korean males than in Korean females. Previous studies also reported a higher prevalence of prediabetes in males [16,23,24]. Sex differences in the prevalence of impaired fasting glucose were associated with biological, psychosocial, and health behavior factors [25]. In previous studies, in addition to the common non-modifiable biological risk factors (e.g., age, family history), males had more lifestyle-related risk factors such as smoking and binge alcohol drinking than females [16,26].
- Similarly, the current study showed that a current or past smoking habit, low-intensity physical activity, and prolonged sedentary behaviors are associated with a higher risk of prediabetes in Korean males only. In previous epidemiological studies, smoking was also associated with the development of type 2 diabetes [27,28]. The nicotine in cigarettes binds to nicotinic acetylcholine receptors in neuronal and non-neuronal or visceral organs, and these receptors participate in signaling within metabolic tissues (e.g., pancreatic islets, adipose tissue) [29]. Thus, nicotine exposure might lead to a proinflammatory metabolic state that could affect insulin sensitivity and beta-cell function [29]. In addition, Zoli and Picciotto [30] suggested that smoking might be associated with adverse fat distribution, including abdominal obesity, which contributes to worse glucose intolerance and insulin sensitivity. According to a cohort study of Korean adults [31], smoking was significantly associated with an increased probability of treatment for type 2 diabetes in both males and females. The risk increased in a dose-dependent manner as the amount of cumulative smoking increased [31]. Furthermore, the cumulative dose-dependent influence of smoking on the development of type 2 diabetes continued after smoking cessation, although smoking cessation had a beneficial effect on reducing the risk of type 2 diabetes [32]. Because the smoking prevalence in Korea is higher in males and females, a current and past smoking history might be more significantly associated with the development of prediabetes in males and females. Similarly, in a previous study of Koreans in their 20s–30s, current smoking was associated with prediabetes in males only. In this context, smoking prevention and smoking cessation should be emphasized as a means of preventing prediabetes in adults, especially for males who have a higher prevalence of smoking.
- Physical activity showed an inverse association with insulin resistance as a fundamental contributor to impaired fasting glucose in individuals with or without prediabetes [33]. According to a systematic review, physical activity was effective in improving oral glucose tolerance in individuals with prediabetes [34]. In addition, a meta-analysis of randomized trial studies showed that physical activity had a beneficial effect on reducing fasting blood glucose and HbA1c levels in healthy individuals as well as individuals with prediabetes and type 2 diabetes [35]. Robles-Ordaz et al. [24] reported the preventive effects of physical activity on prediabetes in males and females aged >20 years. However, the beneficial effects of physical activity on insulin sensitivity were dose-dependent, with a combination of intensity, duration, and frequency [36]. Bird and Hawley [37] reported that moderate-intensity physical activity of ≥30 minutes per day for 3 to 5 days a week was associated with improved insulin sensitivity and glycemic control. Moreover, they suggested that repeated regular physical activity produced beneficial long-term effects on insulin sensitivity [37]. Males are more likely to participate in physical activities such as exercise, leisure, and social activities than females. In Korea, this may be associated with the traditional Confucian Korean culture, which recommends differing types of activity for males and females. From an early age, Korean males mainly participate in dynamic physical activities, while females are more involved in static sedentary behaviors. Thus, the association between activity levels and prediabetes might be more significant for males than for females. In a 10-year longitudinal study of Chinese adults who have similar cultural backgrounds, increased physical activity was effective in resolving prediabetes and restoring normal glucose levels in males only [38]. Regular moderate and vigorous physical activity should be encouraged to prevent prediabetes among adults.
- According to a systematic review [39], increased sitting time may also result in the increased occurrence of type 2 diabetes. Hamilton et al. [40] reported that the contractile activity of skeletal muscle has a critical influence on the development of type 2 diabetes. Similarly, objective sedentary time and insulin sensitivity were inversely associated [41]. Sedentary behaviors include sitting and reclining positions such as during TV watching and might be associated with eating more snacks during a time when metabolic activities are at resting levels [42,43]. Thus, prolonged sedentary behavior during waking time can result in increased adiposity (BMI) from increased calorie intake and reduced energy metabolism. According to Kautzky-Willer et al. [44], increasing adiposity resulted in reduced insulin sensitivity in both males and females, although females tended to have better insulin sensitivity than males. They also proposed that sex hormones (estrogen) might demonstrate antidiabetic effects [44]. Therefore, even when increased adiposity is associated with prolonged sedentary behavior, males might show a worse decrease in insulin sensitivity than females. In this context, sedentary behaviors should be reduced to prevent prediabetes among adults with increased insulin resistance. Replacing 30 minutes of sitting time with low-intensity activity improved insulin sensitivity by 5% in individuals with a greater risk of type 2 diabetes [45]. Physical activity, even low-intensity physical activity, should replace sedentary behavior to help prevent prediabetes. Based on the results of this study, males tended to have more risky health behaviors associated with prediabetes. To develop sex-specific intervention strategies for the prevention of prediabetes, healthcare providers need to be aware of the development of prediabetes in males and be able to detect the risky health behaviors associated with prediabetes.
- Individuals with high education levels have greater access to health-related resources that provide information and assistance in following a healthy lifestyle [46]. Despite the association of lower education levels to increased glucose intolerance in males, the effect of educational inequality (lower education levels in females) on glucose intolerance in females was much more significant in a previous study [46]. Furthermore, educational inequality among individuals with glucose intolerances such as type 2 diabetes was more prominent in Korean and Chinese females than Korean and Chinese males, regardless of socioeconomic status [47,48]. Traditionally, Korean society has regarded males as being superior to females, and education was prioritized accordingly, although this males superiority was a stronger influence decades ago. Therefore, elderly Korean females had lower education levels compared to males of the same age. Accordingly, in a previous study of adults aged >45 years from China, a culture similar to Korea’s, data showed that educational inequality was more prevalent in females than in males [46]. Data also revealed that females with low education levels tended not to follow the behavioral guidelines for obesity prevention [46] and showed maladaptive reactions to health information for the prevention of CVDs, ultimately rejecting the health promotion behaviors meant to prevent CVDs [49]. To encourage and promote healthy behaviors among individuals with low education levels, sufficient support should be provided to help these individuals successfully incorporate the health information into their lifestyles.
- Age and a family history of type 2 diabetes have often been associated with prediabetes and type 2 diabetes [25,50], and age is a strong determinant of type 2 diabetes [25]. In one study, the prevalence of type 2 diabetes increased with age up to approximately 65 years, with no significant change in prevalence in later ages [51]. Similarly, according to a previous study conducted in adults aged 35 to 64 years in China and Sweden, the prevalence of prediabetes gradually increased with age in males and females [25]. In addition, in a previous meta-analysis, individuals with a family history of type 2 diabetes showed a 1.4-fold increased risk of prediabetes [50]. Thus, Wagner et al. [50] proposed that a family history of type 2 diabetes might be linked to hepatic insulin resistance, and Katulanda et al. [52] proposed that a family history might reflect genetic vulnerability as a meaningful predictor and assessment tool for prediabetes and type 2 diabetes. Finally, older individuals and those with a family history of type 2 diabetes should be routinely screened for prediabetes as a primary risk group for prediabetes.
- Using national data, this study identified sex differences in the factors associated with prediabetes in Korean adults. However, this study had several limitations. First, because we used a cross-sectional design, a causal association between prediabetes and the potentially associated factors was not confirmed. Therefore, longitudinal cohort studies are required. Second, although the age of the participants ranged from the 20s to 60s, this study did not identify the factors associated with prediabetes according to age. Thus, future studies should report age differences among the factors associated with prediabetes. Third, in previous studies, ethnic differences were found in the factors associated with prediabetes. Thus, further studies should focus on comparing the factors associated with prediabetes according to race or ethnicity.
Discussion
- The results of this study showed a higher rate of prediabetes in Korean males than in Korean females. Age and a family history of type 2 diabetes were associated with prediabetes in both males and females. However, regarding health behaviors, current and past smoking habits, low-intensity physical activity, and prolonged sedentary behavior were significant factors in male but not in females. Thus, sex-specific prevention strategies for prediabetes should be developed, and older individuals and those with a family history of type 2 diabetes should be screened for the risk of prediabetes.
Conclusion
-
Ethics Approval
This study was approved by the Institutional Review Board of Chungnam National University (202111-SB-242-01), was exempted from review because it was a secondary analysis (202111-SB-242-01), and was performed in accordance with the principles of the Declaration of Helsinki.
-
Conflicts of Interest
The author has no conflicts of interest to declare.
-
Funding
This study was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (2021R1A2C100682811).
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
Article information
- 1. DeFronzo RA, Abdul-Ghani M. Assessment and treatment of cardiovascular risk in prediabetes: impaired glucose tolerance and impaired fasting glucose. Am J Cardiol 2011;108(3 Suppl). 3B−24B.ArticlePubMed
- 2. Grundy SM. Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 2012;59:635−43.ArticlePubMed
- 3. Parizadeh D, Rahimian N, Akbarpour S, et al. Sex-specific clinical outcomes of impaired glucose status: a long follow-up from the Tehran Lipid and Glucose Study. Eur J Prev Cardiol 2019;26:1080−91.ArticlePubMed
- 4. Tabak AG, Herder C, Rathmann W, et al. Prediabetes: a high-risk state for diabetes development. Lancet 2012;379:2279−90.ArticlePubMedPMC
- 5. Centers for Disease Control and Prevention (CDC). National diabetes statistics report 2020. Atlanta: CDC; 2020.
- 6. Korean Diabetes Association (KDA). Diabetes fact sheet 2020. Seoul: KDA; 2020. Korean.
- 7. van Herpt TT, Ligthart S, Leening MJ, et al. Lifetime risk to progress from pre-diabetes to type 2 diabetes among women and men: comparison between American Diabetes Association and World Health Organization diagnostic criteria. BMJ Open Diabetes Res Care 2020;8:e001529.ArticlePubMedPMC
- 8. Einarson TR, Acs A, Ludwig C, et al. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol 2018;17:83. ArticlePubMedPMC
- 9. Gillies CL, Abrams KR, Lambert PC, et al. Pharmacological and lifestyle interventions to prevent or delay type 2 diabetes in people with impaired glucose tolerance: systematic review and meta-analysis. BMJ 2007;334:299. ArticlePubMedPMC
- 10. Vatcheva KP, Fisher-Hoch SP, Reininger BM, et al. Sex and age differences in prevalence and risk factors for prediabetes in Mexican-Americans. Diabetes Res Clin Pract 2020;159:107950. ArticlePubMed
- 11. Amiri P, Jalali-Farahani S, Karimi M, et al. Factors associated with pre-diabetes in Tehranian men and women: a structural equations modeling. PLoS One 2017;12:e0188898.ArticlePubMedPMC
- 12. Hadaegh F, Derakhshan A, Zafari N, et al. Pre-diabetes tsunami: incidence rates and risk factors of pre-diabetes and its different phenotypes over 9 years of follow-up. Diabet Med 2017;34:69−78.ArticlePubMed
- 13. Sadeghi M, Talaei M, Parvaresh Rizi E, et al. Determinants of incident prediabetes and type 2 diabetes in a 7-year cohort in a developing country: the Isfahan cohort study. J Diabetes 2015;7:633−41.ArticlePubMed
- 14. Siddiqui S, Zainal H, Harun SN, et al. Gender differences in the modifiable risk factors associated with the presence of prediabetes: A systematic review. Diabetes Metab Syndr 2020;14:1243−52.ArticlePubMed
- 15. Ghasemi A, Tohidi M, Derakhshan A, et al. Cut-off points of homeostasis model assessment of insulin resistance, beta-cell function, and fasting serum insulin to identify future type 2 diabetes: Tehran Lipid and Glucose Study. Acta Diabetol 2015;52:905−15.ArticlePubMed
- 16. Diaz-Redondo A, Giraldez-Garcia C, Carrillo L, et al. Modifiable risk factors associated with prediabetes in men and women: a cross-sectional analysis of the cohort study in primary health care on the evolution of patients with prediabetes (PREDAPS-Study). BMC Fam Pract 2015;16:5−13.ArticlePubMedPMC
- 17. Pham NM, Eggleston K. Prevalence and determinants of diabetes and prediabetes among Vietnamese adults. Diabetes Res Clin Pract 2016;113:116−24.ArticlePubMed
- 18. Stein KV, Rieder A, Dorner TE. East-West gradient in cardio-vascular mortality in Austria: how much can we explain by following the pattern of risk factors? Int J Health Geogr 2011;10:59. ArticlePubMedPMC
- 19. Korean Diabetes Association (KDA). Diabetes fact sheet 2018. Seoul: KDA; 2018. Korean.
- 20. World Health Organization (WHO). Global physical activity questionnaire (GPAQ) analysis guide (version 2.0). Geneva: WHO; 2019.
- 21. Son N, Sung H, Kim Y. The association between the levels of sedentary time, physical activity, and obesity in Korean older adults. Korean J Sports Med 2021;39:60−7. Korean.Article
- 22. Kim MK, Lee WY, Kang JH, et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul) 2014;29:405−9.ArticlePubMedPMC
- 23. Chandrupatla SG, Khalid I, Muthuluri T, et al. Diabetes and prediabetes prevalence among young and middle-aged adults in India, with an analysis of geographic differences: findings from the National Family Health Survey. Epidemiol Health 2020;42:e2020065.PubMedPMC
- 24. Robles-Ordaz MD, Gallegos-Aguilar AC, Urquidez-Romero R, et al. Prevalence of prediabetes and modifiable factors in an ethnic group of Mexico: the Comcaac Project. Public Health Nutr 2018;21:333−8.ArticlePubMed
- 25. Zhang Y, Santosa A, Wang N, et al. Prevalence and the association of body mass index and other risk factors with prediabetes and type 2 diabetes among 50,867 adults in China and Sweden: a cross-sectional study. Diabetes Ther 2019;10:2061−77.ArticlePubMedPMC
- 26. Park KS, Hwang SY. Lifestyle-related predictors affecting prediabetes and diabetes in 20-30-year-old young Korean adults. Epidemiol Health 2020;e2020014. ArticlePubMedPMC
- 27. Saeed AA. Association of tobacco products use and diabetes mellitus-results of a national survey among adults in Saudi Arabia. Balkan Med J 2012;29:247−51.ArticlePubMedPMC
- 28. Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654−64.ArticlePubMed
- 29. Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res 2017;184:101−7.ArticlePubMedPMC
- 30. Zoli M, Picciotto MR. Nicotinic regulation of energy homeostasis. Nicotine Tob Res 2012;14:1270−90.ArticlePubMedPMC
- 31. Jee SH, Foong AW, Hur NW, et al. Smoking and risk for diabetes incidence and mortality in Korean men and women. Diabetes Care 2010;33:2567−72.ArticlePubMedPMC
- 32. Park SE, Seo MH, Cho JH, et al. Dose-dependent effect of smoking on risk of diabetes remains after smoking cessation: a nationwide population-based cohort study in Korea. Diabetes Metab J 2021;45:539−46.ArticlePubMedPMC
- 33. Dube JJ, Amati F, Toledo FG, et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 2011;54:1147−56.ArticlePubMedPMC
- 34. Jadhav RA, Hazari A, Monterio A, et al. Effect of physical activity intervention in prediabetes: a systematic review with meta-analysis. J Phys Act Health 2017;14:745−55.ArticlePubMed
- 35. Boniol M, Dragomir M, Autier P, et al. Physical activity and change in fasting glucose and HbA1c: a quantitative meta-analysis of randomized trials. Acta Diabetol 2017;54:983−91.ArticlePubMed
- 36. Dube JJ, Allison KF, Rousson V, et al. Exercise dose and insulin sensitivity: relevance for diabetes prevention. Med Sci Sports Exerc 2012;44:793−9.PubMedPMC
- 37. Bird SR, Hawley JA. Update on the effects of physical activity on insulin sensitivity in humans. BMJ Open Sport Exerc Med 2017;2:e000143.ArticlePubMedPMC
- 38. Song X, Qiu M, Zhang X, et al. Gender-related affecting factors of prediabetes on its 10-year outcome. BMJ Open Diabetes Res Care 2016;4:e000169.ArticlePubMedPMC
- 39. Wilmot EG, Edwardson CL, Achana FA, et al. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 2012;55:2895−905.ArticlePubMed
- 40. Hamilton MT, Hamilton DG, Zderic TW. Sedentary behavior as a mediator of type 2 diabetes. Med Sport Sci 2014;60:11−26.ArticlePubMedPMC
- 41. Lahjibi E, Heude B, Dekker JM, et al. Impact of objectively measured sedentary behaviour on changes in insulin resistance and secretion over 3 years in the RISC study: interaction with weight gain. Diabetes Metab 2013;39:217−25.ArticlePubMed
- 42. Bowman SA. Television-viewing characteristics of adults: correlations to eating practices and overweight and health status. Prev Chronic Dis 2006;3:A38. PubMedPMC
- 43. Kikuchi H, Inoue S, Odagiri Y, et al. Occupational sitting time and risk of all-cause mortality among Japanese workers. Scand J Work Environ Health 2015;41:519−28.ArticlePubMed
- 44. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev 2016;37:278−316.ArticlePubMedPMC
- 45. Yates T, Henson J, Edwardson C, et al. Objectively measured sedentary time and associations with insulin sensitivity: Importance of reallocating sedentary time to physical activity. Prev Med 2015;76:79−83.ArticlePubMed
- 46. Chung GK, Lai FT, Yeoh EK, et al. Gender-specific trends of educational inequality in diagnosed diabetes from 1999 to 2014 in Hong Kong: a serial cross-sectional study of 97,481 community-dwelling Chinese adults. Popul Health Metr 2021;19:37. ArticlePubMedPMC
- 47. Wu H, Bragg F, Yang L, et al. Sex differences in the association between socioeconomic status and diabetes prevalence and incidence in China: cross-sectional and prospective studies of 0.5 million adults. Diabetologia 2019;62:1420−9.ArticlePubMedPMC
- 48. Tran BT, Jeong BY, Oh JK. The prevalence trend of metabolic syndrome and its components and risk factors in Korean adults: results from the Korean National Health and Nutrition Examination Survey 2008-2013. BMC Public Health 2017;17:71. ArticlePubMedPMC
- 49. Chung GKK, Yu RHY, Ho SSY, et al. Associations of consuming specific fruit and vegetable subgroups with LDL-C status in early postmenopausal Chinese women. Menopause 2018;25:436−43.ArticlePubMed
- 50. Wagner R, Thorand B, Osterhoff MA, et al. Family history of diabetes is associated with higher risk for prediabetes: a multicentre analysis from the German Center for Diabetes Research. Diabetologia 2013;56:2176−80.ArticlePubMed
- 51. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650−64.ArticlePubMedPMC
- 52. Katulanda P, Ranasinghe P, Jayawardena R, et al. The influence of family history of diabetes on disease prevalence and associated metabolic risk factors among Sri Lankan adults. Diabet Med 2015;32:314−23.ArticlePubMed
References
Figure & Data
References
Citations

- The effect of a nutrition program based on the Health Behavior Interaction Model on primary school students’ nutritional attitudes and behaviors
Ayşe Burcu Başçı, Oya Nuran Emiroğlu, Bilge Kalanlar
Journal of Public Health.2023;[Epub] CrossRef - Factors associated with the combination of general and abdominal obesity in middle-aged and older Korean women: a cross-sectional study
Jin Suk Ra
Osong Public Health and Research Perspectives.2023; 14(5): 379. CrossRef


 PubReader
PubReader Cite
Cite