Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 13(5); 2022 > Article
-
Review Article
Zika virus as an emerging arbovirus of international public health concern -
Samira Vaziri1
 , Siavash Hamzeh Pour2
, Siavash Hamzeh Pour2 , Fateme Akrami-Mohajeri3,4
, Fateme Akrami-Mohajeri3,4
-
Osong Public Health and Research Perspectives 2022;13(5):341-351.
DOI: https://doi.org/10.24171/j.phrp.2022.0101
Published online: October 12, 2022
1Department of Biology, Payame Noor University, Tehran, Iran
2Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
3Infectious Diseases Research Center, Shahid Sadoghi Hospital, Shahid Sadoghi University of Medical Sciences, Yazd, Iran
4Department of Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- Corresponding author: Fateme Akrami-Mohajeri Infectious Diseases Research Center, Shahid Sadoghi Hospital, Shahid Sadoghi University of Medical Sciences, Alam Square, PO Box: 89151-73160, Yazd, Iran E-mail: Fateme.akrami@gmail.com
© 2022 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
- Zika virus (ZIKV) was identified in 1947 in a rhesus monkey during an investigation of the yellow fever virus in the Zika Forest of Uganda; it was also isolated later from humans in Nigeria. The main distribution areas of ZIKV were the African mainland and South-East Asia in the 1980s, Micronesia in 2007, and more recently the Americas in 2014. ZIKV belongs to the Flaviviridae family and Flavivirus genus. ZIKV infection, which is transmitted by Aedes mosquitoes, is an emerging arbovirus disease. The clinical symptoms of ZIKV infection are fever, headache, rashes, arthralgia, and conjunctivitis, which clinically resemble dengue fever syndrome. Sometimes, ZIKV infection has been associated with Guillain-Barré syndrome and microcephaly. At the end of 2015, following an increase in cases of ZIKV infection associated with Guillain-Barré syndrome and microcephaly in newborns in Brazil, the World Health Organization declared a global emergency. Therefore, considering the global distribution and pathogenic nature of this virus, the current study aimed at reviewing the virologic features, transmission patterns, clinical manifestations, diagnosis, treatment, and prevention of ZIKV infection.
- Zika virus (ZIKV) is an arbovirus transmitted by Aedes species mosquitoes. ZIKV was initially identified in 1947 in a febrile sentinel rhesus monkey in the Zika Forest of Uganda, near Lake Victoria [1–3]. In the beginning of 1948, ZIKV was also detected in Aedes africanus mosquitoes in the same forest [4]. Therefore, the name ZIKV originated from the geographical area where it was initially isolated. The virus is classified in the family Flaviviridae, and it is highly similar to other members of the family, such as the yellow fever virus (YFV), Japanese encephalitis virus (JEV), and West Nile virus (WNV) [5–7].
- Serologic studies have also proven human infection [3]. During studies in the early 1950s, ZIKV was also detected in humans in Nigeria [8]. In 1956, an in vitro study indicated that artificially fed Aedes aegypti mosquitoes can transmit the virus to mice and monkeys [9,10]. Some serologic evidence of human ZIKV infection in several African and Asian countries emerged from 1951 to 1981 [11]. The virus was limited to a narrow African/Asian niche until 2007, when an outbreak in Micronesia was first documented [12]. Further outbreaks also happened in the Pacific Island nations from 2013 to 2014, and since 2015 it has been reported in Central and South America [13–15].
- The symptoms of people infected with ZIKV are usually mild and very general, including increased blood temperature, cutaneous rash, headache/malaise, nonpurulent conjunctivitis, arthralgia, myalgia, and gastrointestinal impairment; the symptoms continue for several days and then disappear [6,15]. The high rates of primary microcephaly and Guillain-Barré syndrome (GBS) associated with ZIKV infection in Brazil have raised concerns that the virus circulating in these regions is a rapidly developing neuropathic, teratogenic, emerging infectious public health threat [7,16]. According to a World Health Organization (WHO) announcement on February 1, 2016, the ZIKV infection outbreak represented a “public health emergency of international concern” (PHEIC) [17]. Based on international health regulations, a PHEIC is an extraordinary global event associated with public health risk, which requires international coordination due to the worldwide spread of a disease [18]. Therefore, considering the global emergence and pathogenic nature of this virus, the current study aimed at reviewing the virologic features, transmission pattern, clinical manifestation, diagnosis, treatment, and prevention of ZIKV infection.
Introduction
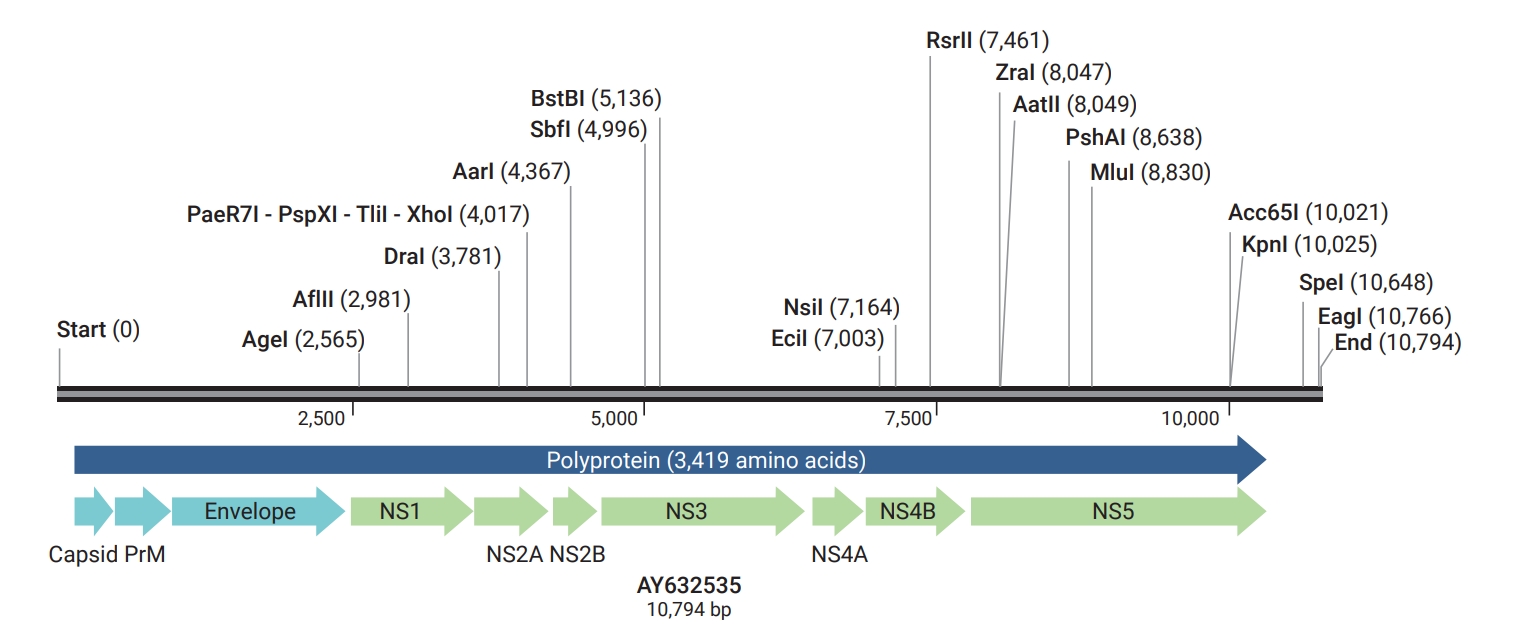
- ZIKV is an arbovirus in the family Flaviviridae, genus Flavivirus, which phylogenetically and antigenically is most closely related to the Spondweni virus. It is an enveloped virus comprising a single-stranded RNA genome with a positive polarity of approximately 11 kb [19,20]. Three lineages have been discovered with the aid of phylogenetic examinations: 1 Asian and 2 African lineages (African I and African II) [21,22]. All lineages originated from the same source in Uganda in the early 20th century [22]. The virus’s RNA includes its complete open reading frame (ORF) sequence. The ORF encodes a polyprotein comprising 3 structural components (capsid [C], premembrane [prM], and envelope [E]) and 7 nonstructural proteins (Table 1; Figure 1) [23–31]. The E and prM proteins are the surface particles of the virus, and the C protein and the genomic RNA molecule make up the nucleocapsid [6,32,33]. Existing information about the pathogenesis of ZIKV is adequate; however, the main immunological and pathological knowledge about the effects of viral proteins on the host is derived from the results of studies on clinically important flaviviruses that tend to cause central nervous system infections; such as dengue virus (DENV), WNV, tick-borne encephalitis virus, JEV, YFV, and St. Louis encephalitis virus [33]. While ZIKV has a significant sequence similarity to the other human flaviviruses, it shows substantial differences in pathology. Clinically, unlike related flaviviruses, such as WNV and DENV, which can infect neural cells but either target mature neurons (WNV) or elicit a less cytotoxic response (DENV), ZIKV exhibits a clear tropism for proliferative neural cells, often with cytotoxic effects [34]. ZIKV can be transferred to humans through mosquito bites, or by injecting the virus into the skin and submucosa of a mammalian host. Therefore, the skin and mucous membranes play an important role in preventing the spread of infection. Human skin cells, such as keratinocytes and fibroblasts, are susceptible to the infection and replication of ZIKV [19].
- Binding of the viral envelope protein and uptake of particles into susceptible cells are necessary for ZIKV infection, which proceeds through specific cell receptors such as AXL, DC SIGN, Tyro3, and TIM-1. ZIKV activates the transcription of Toll-like receptor 3 (TLR3), MDA5, RIG-I, and interferon-stimulated genes such as OAS2, ISG15 and MX1, characterized by strongly enhanced beta-interferon gene expression [19,35]. Recent studies have shown that the depletion of AXL reduces ZIKV infection of cultured fibroblasts and astrocytes [34]. Furthermore, previous studies on developing fetal brain cells in humans observed that these cells are highly enriched with AXL receptors, making them susceptible to ZIKV infection [36]. According to studies on DENV infection, primary ZIKV infection may induce apoptosis in the infected cells, allowing it to evade the innate immune response and boosting the primary release of viral particles [37]. Consequently, both DENV and ZIKV increase replication by autophagy; however, pharmacological modulation was achieved using 3-methyladenine, an autophagosome formation inhibitor that decreases the copy number of viruses in infected fibroblasts [7,19]. ZIKV is not the only flavivirus that triggers apoptosis and autophagy. For example, both DENV and WNV can trigger caspase activation to induce apoptosis [34]. Murine model studies on the inoculation of ZIKV to mouse brains showed that ZIKV-induced autophagy can be assumed to play a pivotal role in the pathogenesis of primary microcephaly [38]. Findings of the Carlos Chagas Institute of Parana Fiocruz indicated that ZIKV can pass through the placenta. The potential mechanism of ZIKV trans-placental transport is that the antibodies generated from a previous flavivirus infection bind to ZIKV particles in the maternal blood and could cross the villous trees to reach the fetal vessels by infecting neonatal Fc receptor (FcRn)-bearing cells [7,39–41]. In vitro neuropathogenesis studies have demonstrated that human neural progenitor cells (hNPCs) of the developing fetus are effectively infected by ZIKV. ZIKV-infected hNPCs can be another explanation for ZIKV infection of the fetal central nervous system, leading to brain abnormalities including microcephaly [42–44]. A recent in vitro study revealed that the expression of 11 microcephaly-related genes (CDK5RAP2, MCPH1, CASC5, WDR62, ASPM, CENPJ/CPAP, STIL, CEP135, CEP152, ZNF335, and PHC1) was reduced in ZIKV-associated microcephaly tissues. All of these genes regulate the cell cycle and cell death, and it is likely that the decreased expression of these genes is an indirect effect of ZIKV infection and that prenatal infection with this virus stops the cell cycle, leading to apoptosis and differentiation defects in the developing nervous system [34].
Virology and Pathogenesis
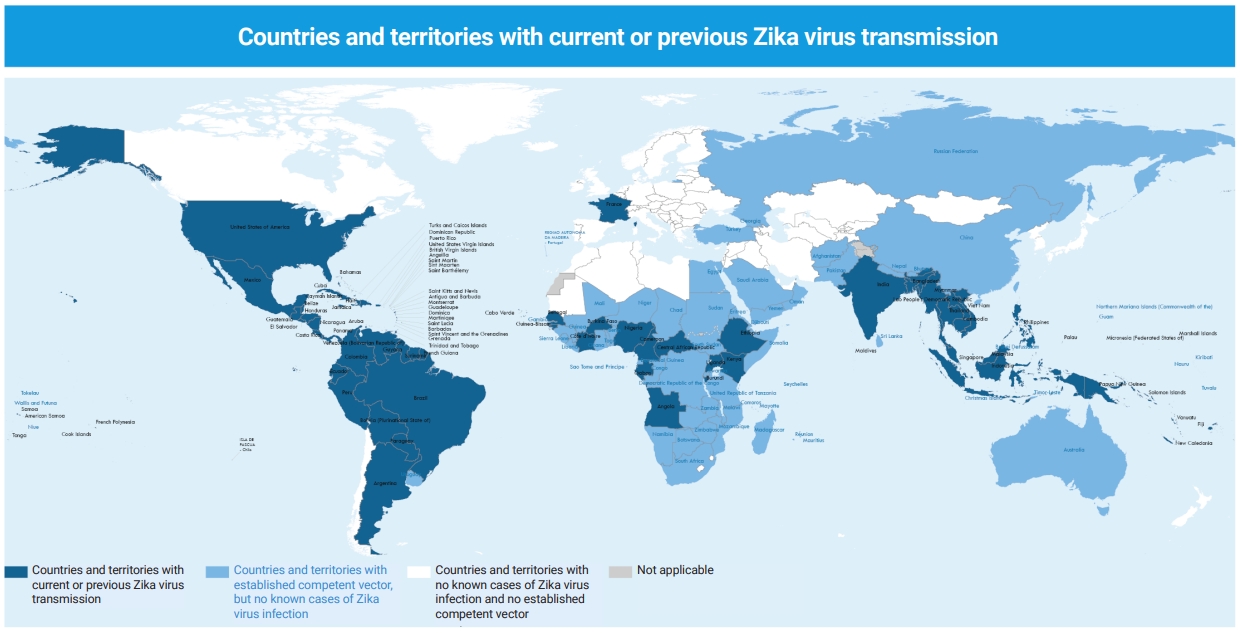
- Primary virological and serological studies indicated that ZIKV infection was limited to African and Asian countries from the 1950s to the 1980s [9,45,46]. Endemic circulation of the virus has been reported in some Asian countries such as Thailand, Indonesia, Peninsular Malaysia, Cambodia, Philippines, and Borneo, as well as in passengers from endemic areas (Table 2) [1–4,8,10–14,17,22,47–50]. Within a 60-year period from 1947 to 2007, Asia and Africa were the only areas with serological and entomological reports [11,51,52]. In 2007, the first outbreak outside of Africa and Asia was reported from Yap Island, Micronesia, in the western Pacific Ocean, north of Papua New Guinea, with 49 confirmed cases based on immunoglobulin M (IgM) seropositivity, and 73% of the residents infected with ZIKV were 3 years of age or older [5,12]. From 2013 to 2014, the Western Pacific islands of French Polynesia, Easter Island, and New Caledonia became the next endemic areas [13]. In March 2015, the first molecularly confirmed case of ZIKV infection was identified in Brazil. The first report of the WHO on ZIKV transmission was published in May 2015 in mainland South America; the possible explanation was that the virus was transmitted during the Va’a World Sprint Championship canoe race in Rio de Janeiro, Brazil, in August 2014 [22]. The phylogeny of the Brazilian strain was similar to that of the French Polynesian one in 2013-2014, both belonging to the Asian lineage. After the May 2015 introduction of the ZIKV in Brazil, it spread rapidly across Brazil and the Americas [47]. On January 28, 2016, 26 countries in the Americas reported autochthonous cases of ZIKV infection and on February 1, 2016, the WHO declared a PHEIC [53]. Outbreaks of ZIKV infection peaked in 2016 and decreased significantly during 2017 and 2018 in the Americas. However, in the South-East Asia Region, in May 2017, 4 ZIKV-positive cases confirmed by reverse transcription-polymerase chain reaction (RT-PCR) were reported from India (Gujarat, 3; Tamil Nadu, 1). From September to November 2018, the largest Indian ZIKV outbreak was reported from the states of Rajasthan and Madhya Pradesh. In total, 159 cases (64 pregnant women) and 130 cases (42 pregnant women) were confirmed as ZIKV-positive by RT-PCR in the states of Rajasthan and Madhya Pradesh, respectively [48]. ZIKV continues to develop and spread silently throughout the world in the form of asymptomatic infections. In July 2021, India reported a ZIKV outbreak in Kerala, the first outbreak activity in South-East Asia Region since the outbreak in Rajasthan, India, in 2018. Extensive testing identified at least 70 PCR-confirmed cases of ZIKV disease by August 2021 [49,50]. Accurate and up-to-date epidemiological data on ZIKV are limited in many parts of the world. Most ZIKV infections are asymptomatic, and if they do occur, symptoms are generally mild and non-specific, and therefore may not be detected or reported. However, as of December 2021, 89 countries and regions have recorded autochthonous mosquito-borne transmission of ZIKV, and it was distributed in all WHO regions except the Eastern Mediterranean Region (Figure 2). The current status of ZIKV transmission and spread indicates that it has the potential to re-emerge as an epidemic [54].
- ZIKV is epizootic and enzootic in African non-human primates, the main natural reservoir. The bite of contaminated A. aegypti and A. albopictus mosquitoes, found in most parts of America (including the United States), is the primary source of transmission to humans. These mosquitoes also transmit chikungunya, DENV, and YFV [55]. The geographic distribution of A. aegypti globally coincides with the areas of DENV transmission. In rare cases, A. albopictus can be involved in DENV epidemics. Other Aedes species such as A. africanus, A. furcifer, A. taylori, and A. luteocephalus are likely vectors in Africa and Asia [56]. Because of the adaptations of mosquitoes to the climatic conditions of urban and rural regions, their omnipresence in many tropical and subtropical countries, anthropophilic behaviors, the invasion of European and North American residential areas by the mosquitoes, and the potential of mosquitoes to carry other arboviruses, the 2 species of A. aegypti and A. albopictus pose the greatest threats to most countries. Aedes species spreading ZIKV live in different countries of the world; therefore, outbreaks can probably spread the infection in currently unaffected countries [18,57].
- An infection occurs when a human is bitten by a contaminated mosquito. In addition to mosquitoes, other routes of ZIKV transmission are possible, although the epidemiologic significance of ZIKV transmission seems unlikely under natural circumstances. It has been suggested that direct transmission can take place from primates to humans through animal bites [55,58]. ZIKV is detectable in the saliva of 19.2% of infected people, although its epidemiologic features should be established [59]. Sexual transmission has been recorded, and the presence of viral RNA was detected using PCR in semen up to 60 days from the occurrence of clinical symptoms, indicating that the virus could persist in the male genitalia for much longer than in the peripheral blood [60–62]. Perinatal and congenital infections can also happen. The possibility of transmission through transplantation and transfusion is a further major clinical and public health concern [63].
Epidemiology and Transmission
- The clinical manifestation of ZIKV infections is non-specific and often mistaken for other flaviviral infections such as DENV and chikungunya. ZIKV and DENV coexist in many developing countries, where there is limited access to health resources and virus-specific diagnostics are not readily available. The co-circulation of both viruses poses challenges for healthcare providers in differentiating between the 2 infections. These infections have similar clinical features, including fever, conjunctivitis, maculopapular rash (which can be pruritic), and joint pain; which usually appear within about 2 to 12 days after the mosquito bite. The clinical manifestations of the disease may last from several days to a week [33,64]. Early differentiation of ZIKV and DENV infections is important for the prognosis and subsequent monitoring and follow-up of these patients. Although ZIKV infection is self-limiting, in contrast, severe DENV infection leads to a debilitating disease that can cause capillary leakage, electrolyte imbalances, organ impairment, and in some cases death [65]. A study in Singapore showed that patients with DENV infection had significant thrombocytopenia, transaminitis, and monocytosis. Conversely, ZIKV patients had normal platelet, aminotransaminase, and monocyte levels, which can help distinguish DENV from ZIKV [66]. ZIKV only affects one-fifth of the population, and hospitalization-requiring severe disease and death are rare; the signs and symptoms of the disease are relatively mild [67]. The main clinical manifestations during the Yap outbreak were a maculopapular rash (90%), arthritis/arthralgia (65%), fever (65%), conjunctivitis (55%), myalgia (48%), headache (45%), retro-orbital pain (39%), edema (19%), and vomiting (10%) [12]. There is a possible association between maternal ZIKV infection and microcephaly in newborns, as well as developmental ocular abnormalities; in addition, there have been some reports about infected adults with neurologic conditions such as GBS [68]. During the outbreak in French Polynesia, higher rates of GBS were reported; specifically, the incidence of GBS during the outbreak was 20 times higher than in non-outbreak periods [69]. In 62% of Brazilian GBS patients during the outbreak, the ZIKV symptoms were similar. The most remarkable and surprising consequence of ZIKV infection is its possible association with inborn abnormalities, especially microcephaly. Intrauterine or neonatal death is possible. The number of infants with congenital microcephaly became 20 times higher after the epidemic commencement, with over 1,200 cases reported during 2015 (99.7 per 100,000 live births) [33].
Clinical Manifestations
- Specific ZIKV diagnostic algorithms for adults and children have been proposed by the US Centers for Disease Prevention and Control (US-CDC). ZIKV is classified as a human pathogen hazard group 3 and biosafety level 2 microorganism by the UK Advisory Committee on Dangerous Pathogens and the US-CDC, respectively [70,71]. To diagnose ZIKV infection, the routine method is to detect viral RNA in serum samples of cases in the acute phase of the disease using RT-PCR, as well as other specific anti-ZIKV serum antibody tests [72]. The isolation of ZIKV from animals or mosquitoes is another method for diagnosing the disease [72]. The virus can be cultured in some cell lines such as Vero and LLC-MK2, or by intracerebral inoculation of suckling mice [33]. Although these methods can help virological investigations, they are unfeasible in many medical laboratories. Serological tests such as the enzyme-linked immunosorbent assay (ELISA) or immunofluorescence are also used. An IgM antibody response in primary ZIKV-infected patients has been reported; however, a cross-reaction with other flaviviruses may make the diagnosis difficult [72,73]. If the assay result is positive, a plaque-reduction or neutralization test should be conducted to measure virus-specific neutralizing antibodies and discriminate among cross-reacting antibodies produced during primary flavivirus infections [74]. RT-PCR of clinical samples (often the peripheral blood) is the most common laboratory test for diagnosing acute ZIKV infection. RT-PCR is a sensitive method for the accurate differentiation of the ZIKV from other species of flaviviruses with similar manifestations. This is particularly valuable in areas with concurrent circulation of arboviruses as a common phenomenon. Genotyping is also practical for this purpose [75]. After the emergence of the disease, the viremic period can last for 1 to more than 11 days [76]. Based on the Yap outbreak in 2007 in a series of 17 patients, the serum viral load was rather low, ranging from 930 to 728,800 (mean, 21,495) copies/mL [5]. A viral load of 338,797 copies/mL was reported in blood samples collected on day 11 after the incidence of clinical symptoms [5]. Furthermore, saliva, urine, and semen are good specimens to detect ZIKV RNA in some patients, and the sensitivity of RT-PCR for saliva samples is considerably higher than that of blood (57.1% vs. 28.1%) [59,76]. Diagnostic flavivirus infection testing should be performed using acute-phase sera samples collected immediately after the incidence of disease, and the latter samples should also be obtained 2 to 3 weeks after the first blood drainage [10].
Diagnosis
- No vaccines or medications are available to prevent or treat ZIKV infections. There is also no specific antiviral treatment for controlling severe and fatal outbreaks [77]. Treatment is primarily supportive, and symptoms can be generally improved or ameliorated by the aid of fluids, rest and oral sedatives, and antipyretics for reducing fever and pain; however, because of the risk of bleeding, aspirin and other non-steroidal anti-inflammatory drugs can be used only when DENV infection has been excluded [78,79]. Healthcare centers should adequately apply standard precautions as well as further mosquito-proofing actions. Healthcare workers who care for Zika patients are recommended to use insect repellents and carry out mosquito avoidance behaviors. Given that ZIKV causes a range of congenital diseases, including microcephaly in newborns and GBS in adults, it is necessary that women, especially those who wish to become pregnant, be vaccinated. Several different ZIKV vaccine candidates using different platforms have now been developed and tested in preclinical and clinical trials. These include live virus vaccines, viral vector vaccines, inactivated vaccines, nucleic acid vaccines (DNA and RNA vaccines), virus-like particle vaccines, and subunit protein vaccines. Each of these vaccine platforms induces humoral and/or cell-mediated immune responses to varying degrees. Given the current facts, it may be necessary to develop more than 1 type of Zika vaccine. For example, inactivated vaccines are usually safe and could be administered to pregnant women as well as women of childbearing age. However, multiple doses and the use of adjuvants may be required to induce stronger and longer-lasting protection. Live attenuated vaccines can be used in men, children, and the elderly. However, they can induce toxic inflammatory responses by potentially reverting to virulent forms, or may be less effective due to pre-existing immunity to the vector virus. DNA or subunit vaccines are generally considered safe for use in all target populations, including pregnant women. The most advanced vaccine candidates to date include nucleic acid-based vaccines (DNA vaccines) that encode the PrM and E proteins of the H/PF/2013 strain of ZIKV (VRC-ZKADNA090-00-VP and VRCZKADNA085-00-VP) [54,80]. In vitro studies applying small interfering RNA, therapeutic antibodies, and molecules targeting nonstructural proteins (especially NS3 and NS5 proteins) are still in progress, but no antivirals have yet been introduced for specific therapies against flaviviral infections (except hepatitis C virus). There are a few available drugs, including tetracycline, chloroquine, amodiaquine, and mefenamic acid, with in vitro inhibitory effects against flaviviruses (particularly DENV); however, their potential clinical benefits has not yet been confirmed [33,81].
- The WHO reported that about 80% of people in developing countries rely on traditional drugs for their primary health care needs [82]. About 855 traditional medicines used in the world are obtained from crude plant extracts [83]. Medicinal plants are a crucial therapeutic aid for various viral diseases [48,84–90]. Medicinal plants might be effective antimicrobial compounds because they have no or low toxicity. The use of polyphenol-rich medicinal plants and their purified compounds as potential antiviral therapies has been recently explored. Several phytochemical families, including alkaloids, flavonoids, polyphenolics, coumarins, terpenoids, and saponins, have already been described as exerting antiviral activity against numerous enveloped RNA viruses, including flaviviruses. Several studies have shown that a polyphenol from green tea (epigallocatechin gallate) and curcumin inhibit ZIKV and DENV infection. Recent in vitro studies have also shown that Doratoxylon apetalum and Psiloxylon mauritianum extracts prevented ZIKV and DENV infection by inhibiting the entry of the virus into human cells [91–96].
Treatment
- The primary measure to prevent and control ZIKV infection is to avoid the bites of virus-infected mosquitoes. To control ZIKV infection, the following measures should be taken: the use of insect repellent, careful hand hygiene, protection of mucous membranes, wearing shirts with long sleeves and long pants impregnated with permethrin, prevention and elimination of standing water bodies with the risk of mosquitoes’ laying eggs, reduction of outdoors activities overlapping with the time of maximum biting activity of mosquitoes, installing window and door screens, and the application of certain mosquito control programs [97–99]. A cohort study in southern Puerto Rico that began in 2018 examined risk factors for ZIKV-related infection. Among 4,035 participants tested for ZIKV, 651 (16%) had evidence of a recent infection. Infection prevalence increased with older age, from 7% among 1- to 10-year-olds up to 19% among 41- to 50-year-olds. Males had a higher risk of Zika infection prevalence than females. The prevalence of ZIKV infection also decreased with the presence of home screens and air conditioning [100]. The European Centre for Disease Prevention and Control and the US-CDC recommend avoiding traveling to virus-infected areas for pregnant mothers and those who are planning pregnancy. Sex education and the use of condoms are also important to prevent sexually transmitted diseases. Considering that the risk of sexual transmission of ZIKV is relatively high, visitors to endemic areas are recommended to maintain sexual abstinence or practice safe sex [18,98,99].
- Since there is no information about the duration of viremia and viral shedding in bodily fluids such as semen, urine, and saliva, it is also not clear how long precautions should be considered for further empirical verification. Some studies suggested the use of contraceptive penile caps 28 days after returning from endemic areas for asymptomatic travelers, and 6 months after recovery if the patient was still symptomatic [33]. Based on CDC recommendations, protection should be taken during vaginal, anal, and oral intercourse with male partners via proper preventive measures during travel and after returning from endemic regions; in particular, pregnant women are advised to abstain from sex with men who have returned from endemic regions [34,98]. Furthermore, some reports have described the transmission of ZIKV via blood transfusion. Thus, as an effective preventive measure, individuals who have recently returned from ZIKV-affected areas should be prevented from blood and organ donation. The safe disposal of sharp objects contaminated with blood and body fluids in medical centers is another infection control measure. It is particularly important to clean and sterilize non-disposable medical instruments after each use [20].
Prevention
- ZIKV, an arthropod-borne flavivirus, is an emerging infectious disease with the potential to cause a serious global threat to public health. ZIKV infection typically causes a mild and self-limiting disease in otherwise healthy people. However, if ZIKV infection occurs during pregnancy, it can cause fetal abnormalities. Therefore, according to recent PHEIC declarations by the WHO, ZIKV is unique in its ability to lead to congenital anomalies. At present, serologic (ELISA) and molecular (RT-PCR) testing are used to diagnose ZIKV infection. No specific drug or vaccine is available for ZIKV infection, and treatment is supportive based on reducing the symptoms of the disease. Given the current facts, it may be necessary to develop more than 1 type of Zika vaccine. For example, an inactivated vaccine would be needed for pregnant women and perhaps women of childbearing age, while a live attenuated vaccine could be used in men, children and the elderly. DNA or subunit vaccines may be safer and lead to longer immunity. Still, protection against mosquito bites is the most effective way to prevent ZIKV infection.
Conclusion
-
Ethics Approval
None.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
All data generated or analyzed during this study are included in this published article.
-
Authors’ Contributions
Conceptualization and design: SV, SHP, FAM; Data curation: SV, SHP; Supervision: FAM; Validation: SHP, FAM; Visualization: SV, SHP; Writing-original draft: all authors; Writing review & editing: all authors; Final approval of the version to be published: all authors.
Article information
| Proteins | Functions / Possible effects on the host | References |
|---|---|---|
| C | Interaction with RNA for nucleocapsid assembly/Protein C is involved in post-transcriptional regulation in host cells and virus-associated neurological diseases. In addition, ZIKV C protein can form a stable complex with Ras-GAP SH3 domain-binding protein 1 (G3BP1) and caprin-1 to block the formation of stress granules in the host cell; in human neural progenitor cells, it induces ribosomal stress and apoptosis. | [23,24] |
| PrM | Consolidation, contribution to the E protein folding and releasing process/Possible related factor in severe dengue virus pathogenicity, increasing the immature virions’ infectious effect because of anti-PrM antibodies. It also induces cellular oxidative stress and autophagy leading to cell death. | [25] |
| E | Receptor attachment protein, fusion protein/Main protein for inducing neutralizing antibodies against virus infection. | [24,26] |
| NS1 | RNA replication/Transfer to host cell membrane and release into the extracellular space, innate immune system signaling pathways regulator; anti-NS1 antibodies could damage platelets and endothelial cells; C4 complement antagonist. | [27,28] |
| NS2A | RNA synthesis and viral formation/Host cell death by triggering apoptotic factors, IFN antagonist. In addition, NS2A can directly mediate the degradation of adhesion junction proteins, thereby destroying mammalian cortical neurogenesis. | [29] |
| NS2B | Serine protease assembly with NS3/ZIKV NS2B can inhibit the phosphorylation of TBK1 to suppress IFN-β production. | [24,27] |
| NS3 | Serine protease effect in complex with NS2B; possess RNA helicase and triphosphatase activities/Host cell apoptosis, microRNAs modulator, one of the targets of the cytotoxic T cell response, downregulation of the host immune response by inhibiting the induction of IFN and downstream IFN-stimulated genes. | [24,28] |
| NS4A | RNA replication/Human type I IFN signaling pathway blocker, autophagy-inducing factor, anti-death effect on host cells during infection. | [24,30] |
| NS4B | RNA replication/NS4B can combine with TBK1 to inhibit the production of type I IFN; and stress granules modulator. | [24,31] |
| NS5 | Methyl transferase, RNA guanylyl transferase, RNA-dependent RNA polymerase (RdRP), RNA synthesis and capping / Human type I and III IFN signaling pathway blocker, inhibits the production of IFN-β and inhibits the expression of IFN-stimulated genes. | [24,28] |
| Year | Location/country | Event | WHO Regional Office | References |
|---|---|---|---|---|
| 1947–1948 | Uganda | Discovery of ZIKV in febrile sentinel rhesus monkey and isolation of ZIKV from Aedes mosquito | AFRO | [1–4] |
| 1952–1953 | Nigeria, Tanzania, Uganda | Isolation of ZIKV from human | AFRO | [3,8] |
| 1950s–1980s | Bangladesh, Borneo, Burkina Faso, Cameroon, Gabon, Indonesia, India, Malaysia, Philippines, Thailand, etc. | Sporadic cases of ZIKV infection in African and Asian countries | AFRO, SEARO, WPRO | [10,11] |
| 2007–2009 | Yap Island, Micronesia | First outbreak ZIKV outside of Africa and Asia | WPRO | [12] |
| 2013–2014 | Cook Islands, Easter Island, French Polynesia, New Caledonia, etc. | ZIKV outbreak in the Western Pacific Region | WPRO | [13] |
| 2015–2016 | Brazil, Columbia, El Salvador, Mexico, United States of America, Venezuela and Caribbean countries | ZIKV outbreak in the Americas | Region of the Americas/Pan American Health Organization | [14,17,22,47] |
| 2017–2018 | India | Biggest ZIKV outbreak in India | SEARO | [48] |
| 2019 | France (Var department) | First case of autochthonous ZIKV disease in Hyeres, Var department, France | European Region | [49] |
| 2020 | Lao People’s Democratic Republic | One probable case of ZIKV-associated neonatal microcephaly | WPRO | [49] |
| 2021 | India | ZIKV outbreak in India | SEARO | [49,50] |
- 1. Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg 1952;46:509−20.ArticlePubMed
- 2. Kirya BG. A yellow fever epizootic in Zika forest, Uganda, during 1972: part 1. virus isolation and sentinel monkeys. Trans R Soc Trop Med Hyg 1977;71:254−60.ArticlePubMed
- 3. Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg 1952;46:521−34.ArticlePubMed
- 4. Weaver SC, Costa F, Garcia-Blanco MA, et al. Zika virus: history, emergence, biology, and prospects for control. Antiviral Res 2016;130:69−80.ArticlePubMedPMC
- 5. Lanciotti RS, Kosoy OL, Laven JJ, et al. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008;14:1232−9.ArticlePubMedPMC
- 6. Turrini F, Ghezzi S, Pagani I, et al. Zika virus: a re-emerging pathogen with rapidly evolving public health implications. New Microbiol 2016;39:86−90.PubMed
- 7. Malone RW, Homan J, Callahan MV, et al. Zika virus: medical countermeasure development challenges. PLoS Negl Trop Dis 2016;10:e0004530.ArticlePubMedPMC
- 8. Hayes EB. Zika virus outside Africa. Emerg Infect Dis 2009;15:1347−50.ArticlePubMedPMC
- 9. Maharajan MK, Ranjan A, Chu JF, et al. Zika virus infection: current concerns and perspectives. Clin Rev Allergy Immunol 2016;51:383−94.ArticlePubMedPDF
- 10. Hills SL, Fischer M, Petersen LR. Epidemiology of Zika virus infection. J Infect Dis 2017;216(suppl_10):S868−74.ArticlePubMedPMC
- 11. Anderson KB, Thomas SJ, Endy TP. The emergence of Zika virus: a narrative review. Ann Intern Med 2016;165:175−83.ArticlePubMed
- 12. Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009;360:2536−43.ArticlePubMed
- 13. Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis 2014;20:1085−6.ArticlePubMedPMC
- 14. Hennessey M, Fischer M, Staples JE. Zika virus spreads to new areas-region of the Americas, May 2015-January 2016. MMWR Morb Mortal Wkly Rep 2016;65:55−8.ArticlePubMed
- 15. Balkhair A, Al-Maamari K, Alawi FB, et al. Zika virus: a roar after years of whispering. Oman Med J 2016;31:87−8.ArticlePubMedPMC
- 16. European Centre for Disease Prevention and Control (ECDC). Rapid risk assessment: Zika virus epidemic in the Americas: potential association with microcephaly and Guillain-Barré syndrome-10 December 2015. Stockholm: ECDC; 2015.
- 17. Heymann DL, Hodgson A, Sall AA, et al. Zika virus and microcephaly: why is this situation a PHEIC? Lancet 2016;387:719−21.ArticlePubMedPMC
- 18. Petersen E, Wilson ME, Touch S, et al. Rapid spread of Zika virus in the Americas: implications for public health preparedness for mass gatherings at the 2016 Brazil Olympic Games. Int J Infect Dis 2016;44:11−5.ArticlePubMed
- 19. Hamel R, Dejarnac O, Wichit S, et al. Biology of Zika virus infection in human skin cells. J Virol 2015;89:8880−96.ArticlePubMedPMCPDF
- 20. Marano G, Pupella S, Vaglio S, et al. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus 2016;14:95−100.PubMedPMC
- 21. Gong Z, Gao Y, Han GZ. Zika virus: two or three lineages? Trends Microbiol 2016;24:521−2.ArticlePubMed
- 22. Vorou R. Zika virus, vectors, reservoirs, amplifying hosts, and their potential to spread worldwide: what we know and what we should investigate urgently. Int J Infect Dis 2016;48:85−90.ArticlePubMed
- 23. Shang Z, Song H, Shi Y, et al. Crystal structure of the capsid protein from Zika virus. J Mol Biol 2018;430:948−62.ArticlePubMed
- 24. Guo M, Hui L, Nie Y, et al. ZIKV viral proteins and their roles in virus-host interactions. Sci China Life Sci 2021;64:709−19.ArticlePubMedPMCPDF
- 25. Nambala P, Su WC. Role of Zika virus prM protein in viral pathogenicity and use in vaccine development. Front Microbiol 2018;9:1797. ArticlePubMedPMC
- 26. Giraldo MI, Xia H, Aguilera-Aguirre L, et al. Envelope protein ubiquitination drives entry and pathogenesis of Zika virus. Nature 2020;585:414−9.ArticlePubMedPMCPDF
- 27. Xia H, Luo H, Shan C, et al. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat Commun 2018;9:414. ArticlePubMedPMCPDF
- 28. Lee I, Bos S, Li G, et al. Probing molecular insights into Zika virus-host interactions. Viruses 2018;10:233. ArticlePubMedPMC
- 29. Yoon KJ, Song G, Qian X, et al. Zika-virus-encoded NS2A disrupts mammalian cortical neurogenesis by degrading adherens junction proteins. Cell Stem Cell 2017;21:349−58.ArticlePubMedPMC
- 30. Ma J, Ketkar H, Geng T, et al. Zika virus non-structural protein 4A blocks the RLR-MAVS signaling. Front Microbiol 2018;9:1350. ArticlePubMedPMC
- 31. Wu Y, Liu Q, Zhou J, et al. Zika virus evades interferon-mediated antiviral response through the co-operation of multiple nonstructural proteins in vitro. Cell Discov 2017;3:17006. ArticlePubMedPMCPDF
- 32. Blitvich BJ, Firth AE. Insect-specific flaviviruses: a systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015;7:1927−59.ArticlePubMedPMC
- 33. Wong SS, Poon RW, Wong SC. Zika virus infection-the next wave after dengue? J Formos Med Assoc 2016;115:226−42.ArticlePubMed
- 34. Merfeld E, Ben-Avi L, Kennon M, et al. Potential mechanisms of Zika-linked microcephaly. Wiley Interdiscip Rev Dev Biol 2017;6:e273.ArticlePubMedPMCPDF
- 35. Maestre AM, Fernandez-Sesma A. Finding clues for congenital Zika syndrome: Zika virus selective infection of immature neurons. EBioMedicine 2016;10:7−8.ArticlePubMedPMC
- 36. Nowakowski TJ, Pollen AA, Di Lullo E, et al. Expression analysis highlights AXL as a candidate Zika virus entry receptor in neural stem cells. Cell Stem Cell 2016;18:591−6.ArticlePubMedPMC
- 37. Briant L, Despres P, Choumet V, et al. Role of skin immune cells on the host susceptibility to mosquito-borne viruses. Virology 2014;464-465:26−32.ArticlePubMed
- 38. Bell TM, Field EJ, Narang HK. Zika virus infection of the central nervous system of mice. Arch Gesamte Virusforsch 1971;35:183−93.ArticlePubMedPDF
- 39. Adibi JJ, Marques ET Jr, Cartus A, et al. Teratogenic effects of the Zika virus and the role of the placenta. Lancet 2016;387:1587−90.ArticlePubMed
- 40. Driggers RW, Ho CY, Korhonen EM, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 2016;374:2142−51.ArticlePubMed
- 41. Zanluca C, de Noronha L, Duarte Dos Santos CN. Maternal-fetal transmission of the zika virus: an intriguing interplay. Tissue Barriers 2018;6:e1402143.ArticlePubMedPMC
- 42. Bhagat R, Prajapati B, Narwal S, et al. Zika virus E protein alters the properties of human fetal neural stem cells by modulating microRNA circuitry. Cell Death Differ 2018;25:1837−54.ArticlePubMedPMCPDF
- 43. Garcez PP, Loiola EC, Madeiro da Costa R, et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016;352:816−8.ArticlePubMed
- 44. Tang H, Hammack C, Ogden SC, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 2016;18:587−90.ArticlePubMedPMC
- 45. Dasti JI. Zika virus infections: an overview of current scenario. Asian Pac J Trop Med 2016;9:621−5.ArticlePubMed
- 46. Fellner C. Zika virus: anatomy of a global health crisis. P T 2016;41:242−53.PubMedPMC
- 47. Musso D. Zika virus transmission from French Polynesia to Brazil. Emerg Infect Dis 2015;21:1887. Article
- 48. Sharma V, Sharma M, Dhull D, et al. Zika virus: an emerging challenge to public health worldwide. Can J Microbiol 2020;66:87−98.ArticlePubMed
- 49. World Health Organization (WHO). Zika epidemiology update-February 2022 [Internet]. Geneva: WHO; 2021 [cited 2022 Sep 13]. Available from:https://www.who.int/publications/m/item/zika-epidemiology-update---february-2022.
- 50. Yadav PD, Niyas VK, Arjun R, et al. Detection of Zika virus disease in Thiruvananthapuram, Kerala, India 2021 during the second wave of COVID-19 pandemic. J Med Virol 2022;94:2346−9.ArticlePubMedPMCPDF
- 51. Buathong R, Hermann L, Thaisomboonsuk B, et al. Detection of Zika virus infection in Thailand, 2012-2014. Am J Trop Med Hyg 2015;93:380−3.ArticlePubMedPMC
- 52. Alera MT, Hermann L, Tac-An IA, et al. Zika virus infection, Philippines, 2012. Emerg Infect Dis 2015;21:722−4.ArticlePubMedPMC
- 53. Wikan N, Smith DR. Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis 2016;16:e119−26.ArticlePubMed
- 54. Pielnaa P, Al-Saadawe M, Saro A, et al. Zika virus-spread, epidemiology, genome, transmission cycle, clinical manifestation, associated challenges, vaccine and antiviral drug development. Virology 2020;543:34−42.ArticlePubMed
- 55. Chen HL, Tang RB. Why Zika virus infection has become a public health concern? J Chin Med Assoc 2016;79:174−8.ArticlePubMed
- 56. Boyer S, Calvez E, Chouin-Carneiro T, et al. An overview of mosquito vectors of Zika virus. Microbes Infect 2018;20:646−60.ArticlePubMed
- 57. Kraemer MU, Sinka ME, Duda KA, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 2015;4:e08347.ArticlePubMedPMCPDF
- 58. Leung GH, Baird RW, Druce J, et al. Zika virus infection in Australia following a monkey bite in Indonesia. Southeast Asian J Trop Med Public Health 2015;46:460−4.PubMed
- 59. Musso D, Roche C, Nhan TX, et al. Detection of Zika virus in saliva. J Clin Virol 2015;68:53−5.ArticlePubMed
- 60. Nicastri E, Castilletti C, Liuzzi G, et al. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill 2016;21:30314. ArticlePubMedPMC
- 61. Turmel JM, Abgueguen P, Hubert B, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet 2016;387:2501. ArticlePubMed
- 62. Mansuy JM, Suberbielle E, Chapuy-Regaud S, et al. Zika virus in semen and spermatozoa. Lancet Infect Dis 2016;16:1106−7.ArticlePubMed
- 63. Marrs C, Olson G, Saade G, et al. Zika virus and pregnancy: a review of the literature and clinical considerations. Am J Perinatol 2016;33:625−39.ArticlePubMedPMC
- 64. Padilla C, Pan A, Geller A, et al. Zika virus: review and obstetric anesthetic clinical considerations. J Clin Anesth 2016;35:136−44.ArticlePubMed
- 65. Paixao ES, Teixeira MG, Rodrigues LC. Zika, chikungunya and dengue: the causes and threats of new and re-emerging arboviral diseases. BMJ Glob Health 2018;3(Suppl 1):e000530.
- 66. Yan G, Pang L, Cook AR, et al. Distinguishing Zika and Dengue viruses through simple clinical assessment, Singapore. Emerg Infect Dis 2018;24:1565−8.ArticlePubMedPMC
- 67. Martins MM, Medronho RA, Cunha AJ. Zika virus in Brazil and worldwide: a narrative review. Paediatr Int Child Health 2021;41:28−35.ArticlePubMed
- 68. Fauci AS, Morens DM. Zika virus in the Americas: yet another arbovirus threat. N Engl J Med 2016;374:601−4.ArticlePubMed
- 69. Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect 2014;20:O595−6.ArticlePubMed
- 70. Centers for Disease Control and Prevention (CDC). Biosafety in microbiological and biomedical laboratories [Internet]. Atlanta, GA: CDC; 2009 [cited 2022 Sep 13]. Available from: https://www.cdc.gov/labs/pdf/CDC-BiosafetymicrobiologicalBiomedicalLaboratories-2009-P.pdf.
- 71. Advisory Committee on Dangerous Pathogens. The approved list of biological agents. 3rd ed. London: Advisory Committee on Dangerous Pathogens; 2013.
- 72. Noorbakhsh F, Abdolmohammadi K, Fatahi Y, et al. Zika virus infection, basic and clinical aspects: a review article. Iran J Public Health 2019;48:20−31.ArticlePubMedPMC
- 73. Barzon L, Trevisan M, Sinigaglia A, et al. Zika virus: from pathogenesis to disease control. FEMS Microbiol Lett 2016;363:fnw202. ArticlePubMed
- 74. Rabe IB, Staples JE, Villanueva J, et al. Interim guidance for interpretation of Zika virus antibody test results. MMWR Morb Mortal Wkly Rep 2016;65:543−6.ArticlePubMed
- 75. Waggoner JJ, Pinsky BA. Zika virus: diagnostics for an emerging pandemic threat. J Clin Microbiol 2016;54:860−7.ArticlePubMedPMCPDF
- 76. Bingham AM, Cone M, Mock V, et al. Comparison of test results for Zika virus RNA in urine, serum, and saliva specimens from persons with travel-associated Zika virus disease: Florida, 2016. MMWR Morb Mortal Wkly Rep 2016;65:475−8.ArticlePubMed
- 77. Rawal G, Yadav S, Kumar R. Zika virus: an overview. J Family Med Prim Care 2016;5:523−7.ArticlePubMedPMC
- 78. Plourde AR, Bloch EM. A literature review of Zika virus. Emerg Infect Dis 2016;22:1185−92.ArticlePubMedPMC
- 79. Slavov SN, Otaguiri KK, Kashima S, et al. Overview of Zika virus (ZIKV) infection in regards to the Brazilian epidemic. Braz J Med Biol Res 2016;49:e5420.ArticlePubMedPMC
- 80. Pattnaik A, Sahoo BR, Pattnaik AK. Current status of Zika virus vaccines: successes and challenges. Vaccines (Basel) 2020;8:266. ArticlePubMedPMC
- 81. Sahoo M, Jena L, Daf S, et al. Virtual screening for potential inhibitors of NS3 protein of Zika virus. Genomics Inform 2016;14:104−11.ArticlePubMedPMCPDF
- 82. Kaushik S, Dar L, Kaushik S, et al. Anti-dengue activity of super critical extract and isolated oleanolic acid of Leucas cephalotes using in vitro and in silico approach. BMC Complement Med Ther 2021;21:227. ArticlePubMedPMCPDF
- 83. Maridass M, De Britto AJ. Origins of plant derived medicines. Ethnobot Leafl 2008;2008:373−87.
- 84. Sharma V, Kaushik S, Kumar R, et al. Emerging trends of Nipah virus: a review. Rev Med Virol 2019;29:e2010.ArticlePubMedPMCPDF
- 85. Kaushik S, Kaushik S, Sharma Y, et al. The Indian perspective of COVID-19 outbreak. Virusdisease 2020;31:146−53.ArticlePubMedPMCPDF
- 86. Sharma V, Kaushik S, Pandit P, et al. Green synthesis of silver nanoparticles from medicinal plants and evaluation of their antiviral potential against chikungunya virus. Appl Microbiol Biotechnol 2019;103:881−91.ArticlePubMedPDF
- 87. Laughlin CA, Morens DM, Cassetti MC, et al. Dengue research opportunities in the Americas. J Infect Dis 2012;206:1121−7.ArticlePubMedPMC
- 88. Wintachai P, Kaur P, Lee RC, et al. Activity of andrographolide against chikungunya virus infection. Sci Rep 2015;5:14179. ArticlePubMedPMCPDF
- 89. Kaushik S, Kaushik S, Sharma V, et al. Antiviral and therapeutic uses of medicinal plants and their derivatives against dengue viruses. Pharmacogn Rev 2018;12:177−85.Article
- 90. Kaushik S, Dar L, Kaushik S, et al. Identification and characterization of new potent inhibitors of dengue virus NS5 proteinase from Andrographis paniculata supercritical extracts on in animal cell culture and in silico approaches. J Ethnopharmacol 2021;267:113541. ArticlePubMed
- 91. Clain E, Haddad JG, Koishi AC, et al. The polyphenol-rich extract from Psiloxylon mauritianum, an endemic medicinal plant from reunion island, inhibits the early stages of Dengue and Zika virus infection. Int J Mol Sci 2019;20:1860. ArticlePubMedPMC
- 92. Haddad JG, Koishi AC, Gaudry A, et al. Doratoxylon apetalum, an indigenous medicinal plant from Mascarene Islands, is a potent inhibitor of Zika and Dengue virus infection in human cells. Int J Mol Sci 2019;20:2382. ArticlePubMedPMC
- 93. de Castro Barbosa E, Alves TM, Kohlhoff M, et al. Searching for plant-derived antivirals against dengue virus and Zika virus. Virol J 2022;19:31. ArticlePubMedPMCPDF
- 94. Fong YD, Chu JJH. Natural products as Zika antivirals. Med Res Rev 2022;42:1739−80.ArticlePubMedPMCPDF
- 95. Vista FE, Dalmacio LM, Corales LG, et al. Antiviral effect of crude aqueous extracts from ten Philippine medicinal plants against Zika virus. Acta Med Philipp 2020;54:195−202.ArticlePDF
- 96. Rehman A, Ashfaq UA, Javed MR, et al. The screening of phytochemicals against NS5 polymerase to treat Zika virus infection: integrated computational based approach. Comb Chem High Throughput Screen 2022;25:738−51.ArticlePubMedPDF
- 97. Benelli G. Spread of Zika virus: the key role of mosquito vector control. Asian Pac J Trop Biomed 2016;6:468−71.Article
- 98. Centers for Disease Control and Prevention (CDC). Zika basics and how to protect yourself 2018. [Internet]. Atlanta, GA: CDC; 2018 [cited 2022 Sep 13]. Available from: https://www.cdc.gov/zika/pdfs/fs-zika-basics.pdf.
- 99. European Centre for Disease Prevention and Control (ECDC). Factsheet for health professionals 2016 [Internet]. Stockholm: ECDC; 2016 [cited 2022 Sep 13]. Available from: https://www.ecdc.europa.eu/en/zika-virus-infection/facts/factsheet.
- 100. Adams LE, Sanchez-Gonzalez L, Rodriguez DM, et al. Risk factors for infection with chikungunya and Zika viruses in southern Puerto Rico: a community-based cross-sectional seroprevalence survey. PLoS Negl Trop Dis 2022;16:e0010416.ArticlePubMedPMC
References
Figure & Data
References
Citations

- A Review on The Pathogenesis of Cardiovascular Disease of Flaviviridea Viruses Infection
Tie-Hua Yang, Wen-Cong Gao, Xin Ma, Qian Liu, Pan-Pan Pang, Yong-Tang Zheng, Yinnong Jia, Chang-Bo Zheng
Viruses.2024; 16(3): 365. CrossRef - The race against time: Zika virus on the horizon in Pakistan
Moiz Ahmed Khan, Summaiya Zafar
Tropical Doctor.2024;[Epub] CrossRef - Zika virus disease: an alarming situation resurfacing on the radar – a short communication
Sanobar Shariff, Burhan Kantawala, Nakyanzi Hamiidah, Tularam Yadav, Abubakar Nazir, Olivier Uwishema
Annals of Medicine & Surgery.2023; 85(10): 5294. CrossRef



 PubReader
PubReader Cite
Cite