Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(1); 2023 > Article
-
Original Article
The first reported hepatitis E outbreak in a food manufacturing factory: Korea, 2022 -
Hansol Yeom1
 , Soonryu Seo2
, Soonryu Seo2 , Youngsil Yoon2
, Youngsil Yoon2 , Jaeeun Lee3
, Jaeeun Lee3 , Myung-Guk Han4
, Myung-Guk Han4 , Deog-Yong Lee4
, Deog-Yong Lee4 , Sun-Whan Park4
, Sun-Whan Park4 , Song A Park4
, Song A Park4 , Sook-Hyang Jeong5
, Sook-Hyang Jeong5 , Jin Gwack2
, Jin Gwack2
-
Osong Public Health and Research Perspectives 2023;14(1):15-22.
DOI: https://doi.org/10.24171/j.phrp.2022.0305
Published online: February 22, 2023
1Division of Infectious Disease Response, Capital Regional Center for Disease Control and Prevention, Seoul, Korea
2Division of Infectious Disease Control, Bureau of Infectious Disease Policy, Korea Disease Control and Prevention Agency, Cheongju, Korea
3Division of Immunization, Bureau of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agency, Cheongju, Korea
4Division of Viral Disease, Bureau of Infectious Disease Diagnosis Control, Korea Disease Control and Prevention Agency, Cheongju, Korea
5Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- Corresponding author: Jin Gwack Division of Infectious Disease Control, Bureau of Infectious Disease Policy, Korea Disease Control and Prevention Agency, 187 Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju 28159, Korea E-mail: gwackjin@korea.kr
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 1,607 Views
- 134 Download
Abstract
-
Objectives
- On February 16, 2022, 12 cases of hepatitis E virus (HEV) infection were reported in a food manufacturing factory in Korea. The aim of this study was to identify additional cases and to determine the source of this HEV outbreak.
-
Methods
- This study was an in-depth investigation of 12 HEV immunoglobulin M (IgM)-positive cases and their demographic, clinical, and epidemiological characteristics. On-site specimens were collected from the environment and from humans, and a follow-up investigation was conducted 2 to 3 months after the outbreak.
-
Results
- Among 80 production workers in the factory, 12 (15.0%) had acute HEV infection, all of whom were asymptomatic. The follow-up investigation showed that 3 cases were HEV IgM-positive, while 6 were HEV IgG-positive. HEV genes were not detected in the HEV IgM-positive specimens. HEV genes were not detected in the food products or environmental specimens collected on-site. HEV was presumed to be the causative pathogen. However, it could not be confirmed that the source of infection was common consumption inside the factory.
-
Conclusion
- This was the first domestic case of an HEV infection outbreak in a food manufacturing factory in Korea. Our results provide information for the future control of outbreaks and for the preparation of measures to prevent domestic outbreaks of HEV infection.
- Hepatitis E is an acute viral infection caused by the hepatitis E virus (HEV) [1]. According to the World Health Organization, 20 million cases of hepatitis E are reported annually, of which 3.3 million (16.5%) are symptomatic. In 2015, 44,000 people died from hepatitis E [2].
- The incubation period of hepatitis E is 15 to 64 days (average, 40 days) [3]. Hepatitis E is a waterborne and foodborne disease orally transmitted through the consumption of contaminated water or food. The signs and symptoms include fever, fatigue, vomiting, stomachache, jaundice, and dark brown urine. Although these symptoms are like those of hepatitis A, most cases of hepatitis E are asymptomatic. Hepatitis E can progress to chronic infection in immunocompromised patients [1,4].
- HEV has 8 genotypes, 4 (1–4) of which are reported in humans [5]. HEV genotypes 1 and 2 are mostly found in Asian and African countries with poor sanitation and are transmitted through the fecal-to-oral route, often in large-scale waterborne outbreaks. HEV genotypes 3 and 4 cause zoonotic infection between humans and animals, mainly boars, pigs, and deer. Sporadic cases of infection through the consumption of contaminated foods have been reported in Europe, North America, parts of Asia (Japan, Taiwan), Australia, and New Zealand [4,6]. HEV genotype 7 has been reported in people who consume meat and milk derived from camels [7].
- Sporadic outbreaks of hepatitis E from the consumption of undercooked meat and processed meat products have been reported in the developed countries of Europe and Japan [4,8]. Domestic cases of hepatitis E have been reported from the consumption of boar bile and raw roe deer meat [9–11]. As a result, interest in hepatitis E has expanded, and hepatitis E was designated a class 2 notifiable infectious disease with commencement of a mandatory surveillance system on July 1, 2020. According to statistics on infectious diseases by the Korea Disease Control and Prevention Agency (KDCA), approximately 500 cases a year are reported. However, when compared to hepatitis A, B, and C, awareness of hepatitis E is relatively low among healthcare workers and the public in Korea. Studies on hepatitis E are lacking in Korea, which is a non-epidemic area of acute hepatitis E [12].
- On February 16, 2022, an outbreak of hepatitis E was reported in a food manufacturing factory in Korea during a routine health examination of workers. As this was the first reported domestic outbreak of hepatitis E, the KDCA examined the incidence and cause of the outbreak in the factory and initiated an epidemiological investigation to establish preventive measures.
Introduction
- Study Background and Settings
- The site of the outbreak was a food manufacturing factory that produced food by mixing, charging, drying, and packing. Among the 162 employees, 80 were production workers. The processing system in the factory was automated and all operations were sealed. There was no process in which employees had direct contact with the raw materials and products. The production workers worked in 3 shifts (day, evening, and night), and there was a cafeteria used by all employees in the factory. According to the requirements of importing countries, the production workers underwent annual health examinations with a surveillance checklist that included bacillary dysentery, hepatitis A, and hepatitis E. On February 16, 2022, the routine on-site health examination of 80 production workers showed 12 cases of hepatitis E infection.
- Considering that the cases were clustered in one factory during a single time period, and that the number of cases was higher than the usual occurrence reported in the province where the factory was located (4 cases annually), we identified this cluster as an HEV outbreak within the factory.
- Epidemiological Investigation
- According to the diagnostic criteria for hepatitis E, we defined cases as those who tested positive for HEV immunoglobulin M (IgM) among the production workers who worked and underwent routine health examinations in the factory from December 14, 2021 to February 16, 2022. As all cases were asymptomatic, diagnostic test results were substituted for symptoms.
- The cases were detected through routine health examinations and not based on the manifestation of symptoms after consumption of a particular food item. Therefore, the source of common exposure was unclear. The long incubation period of acute hepatitis E (15–64 days) hindered the application of cohort or case-control studies. Therefore, a case series study was conducted involving the production workers who met the case definition.
- Basic case information was obtained using an epidemiologic report form. An in-depth epidemiological investigation was conducted through phone calls. Demographic information was collected including sex, age, and residence, as well as epidemiological characteristics including signs and symptoms; history of underlying disease; history of HEV infection; travel history; history of animal contact; history of contact with HEV-infected patients; history of blood donation, blood transfusion, or organ donation; and history of food consumption during the risk exposure period (15–64 days) and the incubation period for index patients (December 14, 2021 to February 1, 2022). Clinical characteristics were obtained, including HEV test results and alanine transaminase and aspartate transaminase measurements. The history of hospital visits during the estimated exposure period was obtained from the drug utilization review (DUR) data of the Health Insurance Review & Assessment Service. Record reviews were used, since all HEV-positive cases were reportedly asymptomatic.
- Environmental Investigation
- Considering that more than 1 month had passed since the diagnosis of the cases, and that all the cases used the same cafeteria in the factory, the investigation focused more on the cafeteria and food items than on the worksite or environment. The Food Sanitation Act mandates the preservation of food for 144 hours after provision. Since the preserved foods from the risk exposure period were not available, the most recent preserved foods were collected.
- The consumption history of meat and processed meat products from the list of food items provided at the cafeteria was analyzed in addition to the in-depth epidemiological investigation. The consumption history of raw meat, processed meat products, animal liver/intestine, and frozen fruits was examined. Furthermore, the kitchen and cooking environment, method of food preservation and distribution, and supply of food ingredients were investigated by interviewing kitchen employees. Since the IgM-positive HEV infection had occurred in the past, recent changes in suppliers of food ingredients, kitchen workers, and the cooking environment were also investigated.
- Environmental specimens were collected from workroom handles, manufactured products, and water that the cases could have been in contact with while working.
- Laboratory Testing
- Anti-HEV IgM was tested in the positive plasma samples using an abia HEV IgM enzyme linked immunosorbent assay kit (AB Diagnostic Systems GmbH) according to the manufacturer’s manual. Anti-HEV IgM-positive samples were tested for HEV RNA using the PowerChek HEV virus qRT-PCR kit (KogeneBiotech Co.). Primers and probes were designed based on multiple sequence alignment of the HEV genome sequences in the open reading frame 2/3 region. Environmental samples were taken from within the facility, and HEV RNA quantitative reverse transcription polymerase chain reaction (qRT-PCR) tests were performed. Environmental tests were conducted on water purifiers, doorknobs, telephones, manufactured products in the workplace, kitchen tools in the cafeteria, and preserved foods.
- Follow-up Investigation
- To monitor the onset of additional cases and determine the continuation of the outbreak, a follow-up was conducted in April 2022, 2 to 3 months after the outbreak was detected, by screening for HEV IgM, HEV IgG, and HEV genes.
- Data Analysis
- Descriptive statistics (presented as frequencies and percentages) were used to analyze differences in the demographic, clinical, and epidemiological characteristics of the cases collected during the epidemiological investigation. Microsoft Excel 2013 (Microsoft Corp.) was used for the analysis.
- Ethics Approval
- The study protocol was approved by the Institutional Review Board of the KDCA (IRB-2022-08-03-PE-A).
Materials and Methods
Case definition
Study design
Case investigation
Investigation of the cafeteria and food items
Investigation of the worksite and environment
- Descriptive Epidemiology
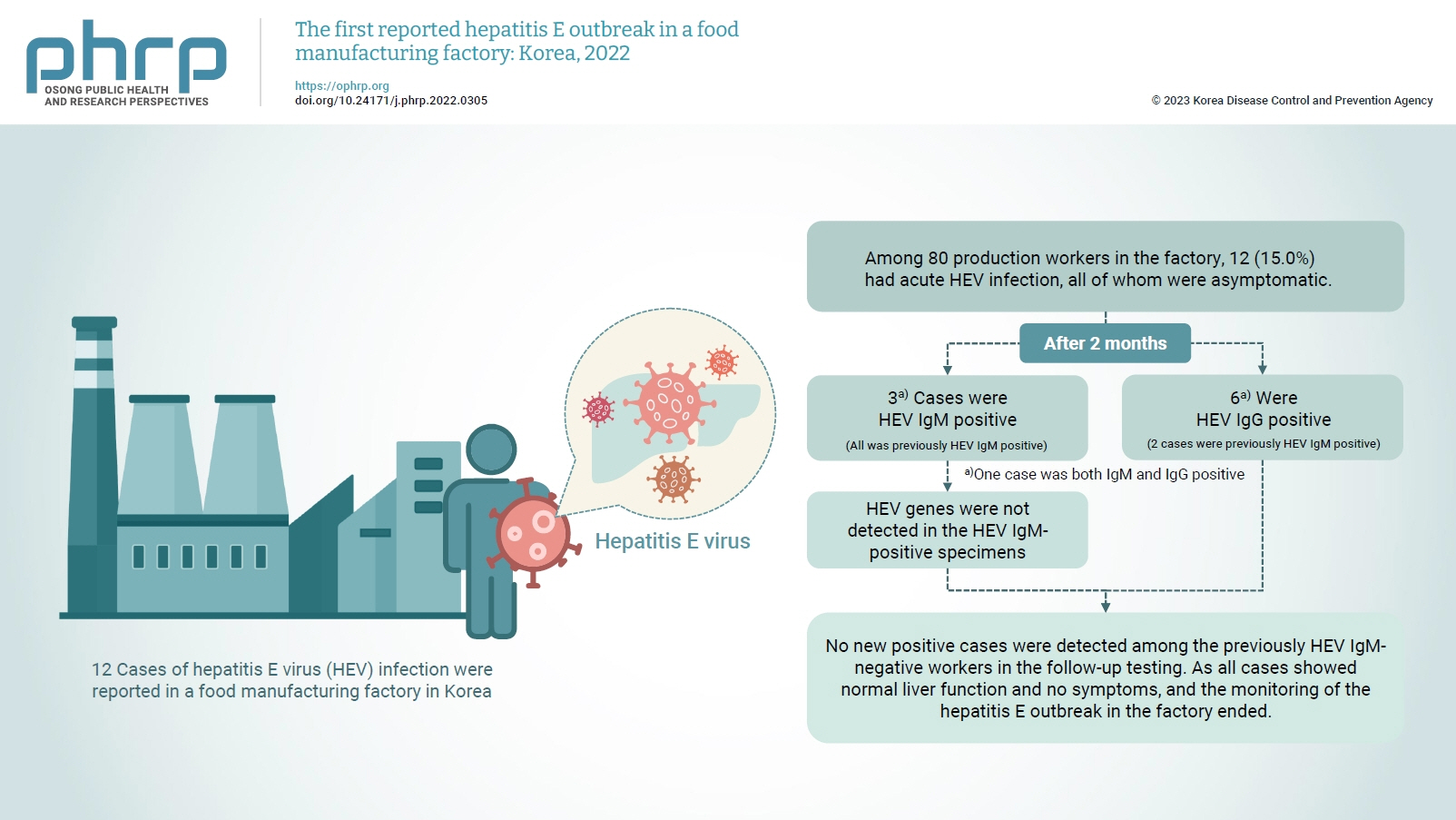
- On February 16, 2022, 12 of 80 production workers in the factory tested positive for HEV in their routine on-site health examinations.
- Demographic and clinical characteristics showed that all 12 cases were men and the median age was 50 years (range, 43–59 years). The attack rate of acute hepatitis E among production workers was 15.0% (12/80). Regarding age, 18.2% of production workers (8/44) were in their 50s and 12.9% (4/31) were in their 40s. The attack rate according to work division was 23.5% (4/17) in department B and 18.8% (3/16) in department E (Table 1).
- All cases were asymptomatic (HEV carriers), and most (10/12) showed normal liver function, based on alanine transaminase and aspartate transaminase levels. A history of chronic diseases (e.g., hypertension) was found in 8 cases, and cases no. 1, no. 3, and no. 8 tested anti-HEV IgM-positive since their previous health examination in 2020. Although all cases reported being asymptomatic during the investigation, the DUR of the Health Insurance Review & Assessment Service revealed that 1 case (no. 8) had presented with digestive symptoms (i.e., gastroenteritis and colitis) during the estimated HEV exposure period (December 2021 to February 2022) (Table 2).
- The analysis of the epidemiological characteristics of the production workers revealed that, within the estimated HEV exposure period, there was no history of overseas travel (including to acute hepatitis E epidemic regions), blood donation, blood transfusion, organ donation, or contact with a hepatitis E-infected patient. As zoonotic infection is possible in acute hepatitis E, any history of animal contact while hunting or while in a barn or farm near the factory or home was examined. The results showed that case no. 12 had a small barn containing 6 hens and 2 dogs. Any history of consuming undercooked meat, animal liver or bile, shellfish, processed meat products (e.g., unheated sausage), or frozen fruits was investigated. Cases no. 4 and no. 10 had consumed cow liver or raw meat but did not develop symptoms (Table 3).
- Environmental Investigation
- During the on-site epidemiological investigation, we collected and tested 3 human specimens from the kitchen employees; 19 environmental specimens from handles in the worksite, kitchen knives, cutting boards, washcloths, preserved food products, and water; and 4 specimens from manufactured products. However, no pathogens were isolated from these specimens, and the source of infection could not be determined. The factory operated its own self-service cafeteria, and no specific problems were identified in the sanitary conditions of the cooking environment, the cooking staff, or the food suppliers. All processes within the workshop were automated and contained within a closed workspace. Therefore, there was no contact between the workers and the products, and the likelihood of contamination of the manufactured food by workers was assessed to be low.
- Follow-up Investigation
- The follow-up tests revealed 3 HEV IgM-positive results (3 previously HEV IgM-positive cases) and 6 HEV IgG-positive results (2 previously HEV IgM-positive cases and 4 previously negative cases). Viral genes were not isolated in the IgM-positive specimens (Table 4). Based on the results of the follow-up investigation, the 3 HEV IgM-positive workers were among those who had first been reported in February 2022, and no new cases had developed among the HEV IgM-negative workers.
Results
- Hepatitis E infection was designated a class 2 notifiable infectious disease in July 2020 in Korea. According to the Health Insurance Review and Assessment Service, the annual number of reported cases of acute hepatitis E was fewer than 100 between 2010 and 2018. Reported cases reached a peak of 219 in 2019, dropped to 169 in 2020, then increased again to 235 in 2021.
- Hepatitis E infection presents a wide range of clinical symptoms, from no or mild symptoms to fulminant hepatitis. Unlike previous reports outside Korea, all cases diagnosed in the current outbreak were asymptomatic [6,13]. However, the predominantly high infection rate in men aged 50 years or older in the present outbreak was in line with previous studies [2,6,14]. This epidemiological investigation showed that all 12 cases of acute hepatitis E from the factory were asymptomatic, but did show HEV IgM-positive results. Therefore, the causative pathogen was presumed to be HEV. Since the virus detected in the human specimens was not detected in the environmental specimens, it could not be determined that the source of infection was through common consumption inside the factory. Furthermore, the epidemiological investigation did not reveal common sources of exposure outside the workplace, such as the use of a common restaurant or other common activities. Acute hepatitis E occurred in 12 of 80 production workers in the factory, accounting for an attack rate of 15.0%. Considering an incubation period of 40 days (range, 15–64 days) from February 2022 when the cases were detected, the infections inside the factory could have occurred between December 2021 and February 2022. Although all cases reported being asymptomatic during the investigation, the DUR of the Health Insurance Review & Assessment Service revealed that 1 case (no. 8) had symptoms of gastroenteritis and colitis and a history of hepatitis E in the past, so the possibility of an index case could not be ruled out. Despite the possibility of co-exposure among all cases, and insufficient evidence for an external environmental route of infection, it was difficult to determine the source of infection.
- To prevent the spread of HEV, we monitored for additional cases and anyone presenting with symptoms for the maximum incubation period of 64 days. In addition, follow-ups were conducted with the production workers 2 to 3 months after the outbreak (April 2022). The local public health center collected blood samples from the production workers between April 18 and 19, 2022, and the Department of Virus Analysis at the KDCA analyzed the specimens. In vitro diagnostic agents approved by the Ministry of Food and Drug Safety were used for antibody testing, and the HEV IgM-positive specimens were subjected to additional viral gene detection tests. Three HEV IgM-positive cases (all previously HEV IgM-positive) and 6 HEV IgG-positive cases (2 previously HEV IgM-positive and 4 previously negative) were found. Among them, 1 case was both HEV IgM- and IgG-positive, and no viral genes were detected in the HEV IgM-positive specimens. The 3 HEV IgM-positive cases had previously tested positive for HEV IgM on February 16, 2022. No new positive cases were detected among the previously HEV IgM-negative workers in the follow-up testing. Although HEV can be detected in blood for 3 to 6 weeks after infection, its detection has also been reported after several months. As all cases showed normal liver function and no symptoms, no further interventions were performed, and the monitoring of the hepatitis E outbreak in the factory ended on April 25, 2022 (Figure S1).
- This outbreak investigation had a few limitations. First, because 19 days passed between the time that a diagnosis of hepatitis E was made and the beginning of the on-site epidemiological investigation and collection of specimens, the timeliness of the investigation, the design, and the selection of subjects were not sufficient. Considering the long incubation time of hepatitis E (15–64 days) and the time when the outbreak was first recognized, the on-site epidemiological investigation was conducted at least 1 month after the onset of infections. Therefore, there were no preserved foods on-site that had been consumed by the HEV IgM-positive cases. It was also difficult to obtain cooperation for the investigation from the factory staff. For this reason, we were only able to investigate HEV IgM-positive production workers and not all employees. Thus, the risk of infection from food consumption could not be determined in this case series study. Although the factory cafeteria was used by all employees, the investigation was only conducted among production workers who were subject to routine health examinations, limiting our assessment of the route and location of the infection in this outbreak. In the event of a future hepatitis E outbreak, an appropriate investigation design and selection of subjects is necessary to identify the source and route of infection and to take effective control measures.
- Second, the factory had failed to take appropriate measures in the past when cases of hepatitis E were found during annual health examinations of the production workers because the cases had no specific symptoms, and the factory was not aware that hepatitis E was a notifiable infectious disease. According to previous studies [15–17], hepatitis E reinfections can occur despite immunization. HEV IgM-positive individuals convert to negative within 6 months on average, but HEV IgM positivity can last for 2 to 3 years. In this case, it was determined that 3 workers had been HEV IgM-positive in the past due to previous exposure to the virus, with antibodies remaining from the previous infection. In the event of an outbreak of hepatitis E, appropriate case management measures require timely notification of the disease.
- Third, the HEV genotype that caused this outbreak could not be determined because qRT-PCR results from the 12 HEV IgM-positive cases did not show viral genes. In a previous study [18], only 1 of 6 cases with HEV IgM-positive results showed positive qRT-PCR results, indicating that HEV viremia had decreased significantly in the serum of cases with acute symptomatic hepatitis E and, therefore, could not be detected. Further efforts are needed to isolate HEV genes from cases.
- Fourth, there is no internationally standardized diagnosis method for hepatitis E, and the possibility of false positives due to the low sensitivity of domestically approved hepatitis E antibody tests cannot be ruled out. According to the results of a previous study in Korea [19], the seroprevalence of HEV IgG in 147 study subjects was 23.1% when tested by Wantai kits (Wantai Biological Pharmacy Enterprise), while the GeneLab (GeneLabs Diagnostics) test for the same group showed 14.3%, indicating a high degree of reproducibility. Therefore, further research is needed on diagnostic methods, including a comparison of the sensitivity and specificity of various hepatitis E antibody test kits.
- Despite these limitations in our investigation, this was the first epidemiological investigation and response to a domestic hepatitis E outbreak in Korea. The results of this study suggest the following strategies for domestic hepatitis E control:
- First, we should raise awareness regarding hepatitis E and provide information and guidance to healthcare workers to enable an early diagnosis when symptoms manifest. Hepatitis E was designated a class 2 notifiable infectious disease in July 2020. However, because its incidence is low in Korea, and most cases are reportedly asymptomatic, awareness is low among healthcare workers. This leads to frequent omissions or delayed reporting, likely resulting from a low rate of diagnosis even when the infection presents with symptoms. To avoid delays in the reporting of hepatitis E, awareness must be raised.
- Second, it is necessary to identify which groups are at risk of infection in Korea to establish effective HEV control strategies. The current outbreak was detected through routine health examinations of production workers required by other countries before exporting the products of the factory. Since hepatitis E is mostly asymptomatic or mildly symptomatic, it is possible to miss hepatitis E even when there is an outbreak. Therefore, it would be helpful to identify domestic risk groups by conducting seroprevalence surveys at the national or regional levels, targeting specific groups with known risk factors.
- Third, since HEV can be transmitted zoonotically, hepatitis E needs to be monitored, prevented, and managed using a One Health approach to prevent the spread of HEV from animal hosts to humans [20]. According to recent studies from Europe, Japan, and Australia, HEV genotypes 3 and 4 can lead to zoonotic waterborne or foodborne infections in humans and animals, mainly pigs, boars, and deer [4,6]. It is necessary to establish a system of communication and collaboration among multiple authorities to prepare an integrated governmental response system for infectious diseases, with a focus on the risk factors among people, animals, food, and the environment.
Discussion
- This was the first domestic outbreak of HEV infection to occur in a food manufacturing factory in Korea. Our results may provide useful information for effective outbreak control and the preparation of preventive measures against future domestic outbreaks of HEV infection. Currently, no commercial vaccine has been developed for hepatitis E in Korea. Therefore, to reduce the prevalence of hepatitis E infection and prevent outbreaks, the importance of consuming foods prepared in sanitary settings and fully cooked at the appropriate temperature should be publicly promoted. Raising public awareness of hepatitis E and establishing supportive systems is vital.
Conclusion
Supplementary Material
Figure S1.
-
Ethics Approval
The study protocol was approved by the Institutional Review Board of the KDCA (IRB-2022-08-03-PE-A). Obtaining informed consent was exempted by the IRB as there was no personal information in the study.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: JG; Data curation: YY, SS, JL, HY; Formal analysis: YY, JL, HY; Investigation: HY, MGH, DYL, SWP, SAP, JL; Methodology: JL, HY, SHJ, JG; Project administration: HY; Resources: HY; Supervision: SS, JG; Visualization: YY, JL, HY; Writing–original draft: HY; Writing–review & editing: all authors. All authors read and approved the final manuscript.
-
Additional Contributions
We are grateful to the investigation participants, the workers, and the managers of the food manufacturing factory for their cooperation. We also thank our colleagues in the related province and the public health center for their contribution to data and sample collection.
Article information
| Characteristic |
Case no. |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Travel history | No | No | No | No | No | No | No | No | No | No | No | No |
| History of blood donation/transfusion | No | No | No | No | No | No | No | No | No | No | No | No |
| Contact with HEV-infected patient | No | No | No | No | No | No | No | No | No | No | No | No |
| Contact with animals | No | No | No | No | No | No | No | No | No | No | No | Yesa) |
| Ingestion of HEV risk-related foods | No | No | No | Yesb) | No | No | No | No | No | Yesc) | No | No |
- 1. Dalton HR, Izopet J, Bendall R. Hepatitis E. In: Sanyal AJ, Boyer, TD, Lindor KD, et al, editors. Zakim and Boyer's hepatology: a textbook of liver disease. 7th ed. Elsevier; 2018. p. 522–34.
- 2. World Health Organization (WHO). Hepatitis E [Internet]. WHO; 2022 [cited 2023 Jan 3]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e.
- 3. Yapa CM, Furlong C, Rosewell A, et al. First reported outbreak of locally acquired hepatitis E virus infection in Australia. Med J Aust 2016;204:274. ArticlePubMedPDF
- 4. Guillois Y, Abravanel F, Miura T, et al. High proportion of asymptomatic infections in an outbreak of hepatitis E associated with a spit-roasted piglet, France, 2013. Clin Infect Dis 2016;62:351−7.ArticlePubMed
- 5. Lampejo T, Curtis C, Ijaz S, et al. Nosocomial transmission of hepatitis E virus and development of chronic infection: the wider impact of COVID-19. J Clin Virol 2022;148:105083. ArticlePubMedPMC
- 6. Zhang L, Yan B, Xu A. A hepatitis E outbreak by genotype 4 virus in Shandong province, China. Vaccine 2016;34:3715−8.ArticlePubMed
- 7. Lee GH, Tan BH, Teo EC, et al. Chronic infection with camelid hepatitis e virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016;150:355−7.ArticlePubMed
- 8. Mizuo H, Yazaki Y, Sugawara K, et al. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol 2005;76:341−9.ArticlePubMed
- 9. Kim YM, Jeong SH, Kim JY, et al. The first case of genotype 4 hepatitis E related to wild boar in South Korea. J Clin Virol 2011;50:253−6.ArticlePubMed
- 10. Choi JY, Lee JM, Jo YW, et al. Genotype-4 hepatitis E in a human after ingesting roe deer meat in South Korea. Clin Mol Hepatol 2013;19:309−14.ArticlePubMedPMC
- 11. Yun H, Kim JS, Lee HJ, et al. The complete genome sequence and molecular analysis of human hepatitis E virus genotype IV identified from a Korean patient. Arch Virol 2010;155:1003−8.ArticlePubMedPDF
- 12. Lim JW, Park CS, Ahn JM, et al. Nine cases of sporadic acute hepatitis E in Korea. Korean J Hepatol 2006;12:230−6. Korean.PubMed
- 13. Yin W, Han Y, Xin H, et al. Hepatitis E outbreak in a mechanical factory in Qingdao City, China. Int J Infect Dis 2019;86:191−6.ArticlePubMed
- 14. Yoon Y, Jeong HS, Yun H. et al. Hepatitis E virus (HEV) seroprevalence in the general population of the Republic of Korea in 2007-2009: a nationwide cross-sectional study. BMC Infect Dis 2014;14:517. PubMedPMC
- 15. Choi Y, Zhang X, Skinner B. Analysis of IgG Anti-HEV antibody protective levels during hepatitis E virus reinfection in experimentally infected rhesus macaques. J Infect Dis 2019;219:916−24.ArticlePubMedPMC
- 16. Riveiro-Barciela M, Rando-Segura A, Barreira-Díaz A, et al. Unexpected long-lasting anti-HEV IgM positivity: is HEV antigen a better serological marker for hepatitis E infection diagnosis? J Viral Hepat 2020;27:747−53.ArticlePubMedPDF
- 17. Lu J, Huang Y, Wang P, et al. Dynamics of hepatitis E virus (HEV) antibodies and development of a multifactorial model to improve the diagnosis of HEV infection in resource-limited settings. J Clin Microbiol 2021;59:e02321−20.ArticlePubMedPMCPDF
- 18. Choi GH, Jeong SH, Hwang JH, et al. Causative role and clinico-epidemiological characteristics of hepatitis E virus in acute viral hepatitis. Public Health Wkly Rep 2021;14:2151−62. Korean.
- 19. Park HK, Jeong SH, Kim JW, et al. Seroprevalence of anti-hepatitis E virus (HEV) in a Korean population: comparison of two commercial anti-HEV assays. BMC Infect Dis 2012;12:142. ArticlePubMedPMCPDF
- 20. Velavan TP, Pallerla SR, Johne R, et al. Hepatitis E: an update on one health and clinical medicine. Liver Int 2021;41:1462−73.ArticlePubMedPDF
References
Figure & Data
References
Citations



 Cite
Cite