Previous issues
- Page Path
- HOME > Articles and issues > Previous issues
Editorial
- What measures should be considered in this 2022-2023 winter season
- Jong-Koo Lee
- Osong Public Health Res Perspect. 2022;13(5):313-315. Published online October 28, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0286
- 1,481 View
- 54 Download
Review Articles
- India’s efforts to achieve 1.5 billion COVID-19 vaccinations: a narrative review
- Kapil Singh, Ashwani Verma, Monisha Lakshminarayan
- Osong Public Health Res Perspect. 2022;13(5):316-327. Published online October 14, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0104
- 3,669 View
- 91 Download
- 8 Web of Science
- 12 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 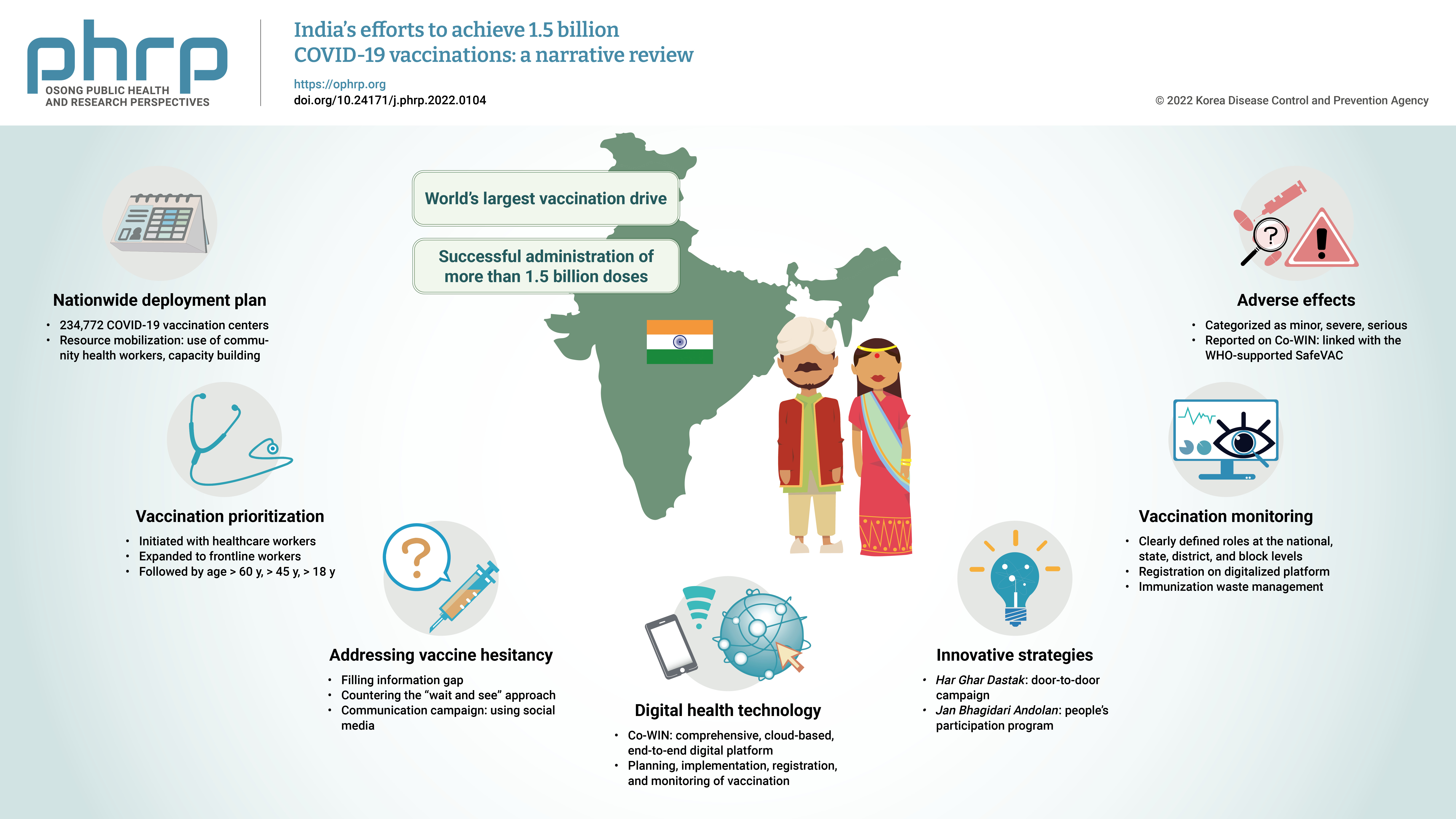
- The initial case of coronavirus disease 2019 (COVID-19) in India was reported on January 30, 2020, and subsequently, the number of COVID-19-infected patients surged during the first wave of April 2020 and the second wave in the same month of 2021. The government of India imposed a strict nationwide lockdown in April 2020 and extended it until May 2020. The second wave of COVID-19 in India overwhelmed the country’s health facilities and exhausted its medical and paramedical workforce. This narrative review was conducted with the aim of summarizing the evidence drawn from policy documents of governmental and non-governmental organizations, as well as capturing India's COVID-19 vaccination efforts. The findings from this review cover the Indian government's vaccination initiatives, which ranged from steps taken to combat vaccine hesitancy to vaccination roadmaps, deployment plans, the use of digital health technology, vaccination monitoring, adverse effects, and innovative strategies such as Har Ghar Dastak and Jan Bhagidari Andolan (people’s participation). These efforts collectively culminated in the successful administration of more than 1.8 billion doses of COVID-19 vaccines in India. This review also provides insights into other countries’ responses to COVID-19 and guidance for future pandemics.
-
Citations
Citations to this article as recorded by- Digital health technology used in emergency large-scale vaccination campaigns in low- and middle-income countries: a narrative review for improved pandemic preparedness
Paula Mc Kenna, Lindsay A. Broadfield, Annik Willems, Serge Masyn, Theresa Pattery, Ruxandra Draghia-Akli
Expert Review of Vaccines.2023; 22(1): 243. CrossRef - Media Reporting Relating to COVID-19 Vaccination as a Driver of Vaccine Hesitancy Prior to the Second Wave of the COVID-19 Pandemic in India: A Content Analysis of Newspaper and Digital Media Reports
Saurav Basu, Himanshi Sharma
Cureus.2023;[Epub] CrossRef - An assessment of the strategy and status of COVID-19 vaccination in India
Sneh Lata Gupta, Surbhi Goswami, Ananya Anand, Namrata Naman, Priya Kumari, Priyanka Sharma, Rishi K. Jaiswal
Immunologic Research.2023; 71(4): 565. CrossRef - Development of a Choice-framework for Covid vaccines in India using a multi-criteria decision analysis approach
Tarun K. George, Nayana P. Nair, Awnish Kumar Singh, A. Dilesh Kumar, Arup Deb Roy, Varshini Neethi Mohan, Gagandeep Kang
Vaccine.2023; 41(25): 3755. CrossRef - COVID-19 Booster Dose Coverage and Hesitancy among Older Adults in an Urban Slum and Resettlement Colony in Delhi, India
Nandini Sharma, Saurav Basu, Heena Lalwani, Shivani Rao, Mansi Malik, Sandeep Garg, Rahul Shrivastava, Mongjam Meghachandra Singh
Vaccines.2023; 11(7): 1177. CrossRef - Review of the unmet medical need for vaccination in adults with immunocompromising conditions: An Indian perspective
Ashok Vaid, Neha Rastogi, T. Mark Doherty, Peter San Martin, Yashpal Chugh
Human Vaccines & Immunotherapeutics.2023;[Epub] CrossRef - Translating the COVID-19 experience in widening the HPV vaccination campaign for cervical cancer in India
Aruni Ghose, Anisha Agarwal, Bhawna Sirohi, Shona Nag, Linus Chuang, Swarupa Mitra
Gynecologic Oncology Reports.2023; 48: 101247. CrossRef - Symptomatic prevalence of covid-19 in vaccinated and non-vaccinated population
Jay Bhupesh Pandya, Nirali Milind Shethia, Divya Bangera, Shailaja Gada Saxena
IP International Journal of Medical Microbiology a.2023; 9(2): 110. CrossRef - Active surveillance of adverse events following COVID-19 vaccines in a tertiary care hospital
Naveena Mary Cherian, Dravya Anna Durai, Muhammed Jaisel, Divyansh Sharma, Juny Sebastian, Chetak Kadabasal Basavaraja, Merrin Mathew
Therapeutic Advances in Vaccines and Immunotherapy.2023;[Epub] CrossRef - Effectiveness of ayurvedic formulation, NAOQ19 along with standard care in the treatment of mild-moderate COVID-19 patients: A double blind, randomized, placebo-controlled, multicentric trial
Pankaj Bhardwaj, Kalaiselvan Ganapathy, Monika Pathania, K.H. Naveen, Jaykaran Charan, Siddhartha Dutta, Ravisekhar Gadepalli, Srikanth Srinivasan, Manoj Kumar Gupta, Akhil D. Goel, Naresh Midha, Bharat Kumar, Meenakshi Sharma, Praveen Sharma, Mithu Baner
Journal of Ayurveda and Integrative Medicine.2023; 14(6): 100778. CrossRef - Balancing Routine and Pandemic: The Synergy of India’s Universal Immunization Program and COVID-19 Vaccination Program
Pawan Kumar, Ashish Birendra Chakraborty, Suhas Dhandore, Pritu Dhalaria, Ajeet Kumar Singh, Disha Agarwal, Kapil Singh, Pretty Priyadarshini, Paras Jain, Vidushi Bahl, Gunjan Taneja
Vaccines.2023; 11(12): 1776. CrossRef - Unveiling vaccine safety: a narrative review of pharmacovigilance in India's COVID-19 vaccination
Megha Hegde, Saurav Raj, Dhananjay Tikadar, Sanatkumar B Nyamagoud
Monaldi Archives for Chest Disease.2023;[Epub] CrossRef
- Digital health technology used in emergency large-scale vaccination campaigns in low- and middle-income countries: a narrative review for improved pandemic preparedness
- Effects of medication adherence interventions for older adults with chronic illnesses: a systematic review and meta-analysis
- Hae Ok Jeon, Myung-Ock Chae, Ahrin Kim
- Osong Public Health Res Perspect. 2022;13(5):328-340. Published online October 12, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0168
- 3,579 View
- 170 Download
-
 Abstract
Abstract
 PDF
PDF - This systematic review and meta-analysis aimed to understand the characteristics of medication adherence interventions for older adults with chronic illnesses, and to investigate the average effect size by combining the individual effects of these interventions. Data from studies meeting the inclusion criteria were systematically collected in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines. The results showed that the average effect size (Hedges’ g) of the finally selected medication adherence interventions for older adults with chronic illnesses calculated using a random-effects model was 0.500 (95% confidence interval [CI], 0.342−0.659). Of the medication adherence interventions, an implementation intention intervention (using face-to-face meetings and telephone monitoring with personalized behavioral strategies) and a health belief model–based educational program were found to be highly effective. Face-to-face counseling was a significantly effective method of implementing medication adherence interventions for older adults with chronic illnesses (Hedges’ g=0.531, 95% CI, 0.186−0.877), while medication adherence interventions through education and telehealth counseling were not effective. This study verified the effectiveness of personalized behavioral change strategies and cognitive behavioral therapy based on the health belief model, as well as face-to-face meetings, as medication adherence interventions for older adults with chronic illnesses.
- Zika virus as an emerging arbovirus of international public health concern
- Samira Vaziri, Siavash Hamzeh Pour, Fateme Akrami-Mohajeri
- Osong Public Health Res Perspect. 2022;13(5):341-351. Published online October 12, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0101
- 2,381 View
- 130 Download
- 3 Web of Science
- 3 Crossref
-
 Abstract
Abstract
 PDF
PDF - Zika virus (ZIKV) was identified in 1947 in a rhesus monkey during an investigation of the yellow fever virus in the Zika Forest of Uganda; it was also isolated later from humans in Nigeria. The main distribution areas of ZIKV were the African mainland and South-East Asia in the 1980s, Micronesia in 2007, and more recently the Americas in 2014. ZIKV belongs to the Flaviviridae family and Flavivirus genus. ZIKV infection, which is transmitted by Aedes mosquitoes, is an emerging arbovirus disease. The clinical symptoms of ZIKV infection are fever, headache, rashes, arthralgia, and conjunctivitis, which clinically resemble dengue fever syndrome. Sometimes, ZIKV infection has been associated with Guillain-Barré syndrome and microcephaly. At the end of 2015, following an increase in cases of ZIKV infection associated with Guillain-Barré syndrome and microcephaly in newborns in Brazil, the World Health Organization declared a global emergency. Therefore, considering the global distribution and pathogenic nature of this virus, the current study aimed at reviewing the virologic features, transmission patterns, clinical manifestations, diagnosis, treatment, and prevention of ZIKV infection.
-
Citations
Citations to this article as recorded by- A Review on The Pathogenesis of Cardiovascular Disease of Flaviviridea Viruses Infection
Tie-Hua Yang, Wen-Cong Gao, Xin Ma, Qian Liu, Pan-Pan Pang, Yong-Tang Zheng, Yinnong Jia, Chang-Bo Zheng
Viruses.2024; 16(3): 365. CrossRef - The race against time: Zika virus on the horizon in Pakistan
Moiz Ahmed Khan, Summaiya Zafar
Tropical Doctor.2024;[Epub] CrossRef - Zika virus disease: an alarming situation resurfacing on the radar – a short communication
Sanobar Shariff, Burhan Kantawala, Nakyanzi Hamiidah, Tularam Yadav, Abubakar Nazir, Olivier Uwishema
Annals of Medicine & Surgery.2023; 85(10): 5294. CrossRef
- A Review on The Pathogenesis of Cardiovascular Disease of Flaviviridea Viruses Infection
Original Articles
- A case-control study of acute hepatitis A in South Korea, 2019
- Jung Hee Hyun, Ju Young Yoon, Sang Hyuk Lee
- Osong Public Health Res Perspect. 2022;13(5):352-359. Published online October 12, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0141
- 2,908 View
- 117 Download
- 1 Web of Science
- 4 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 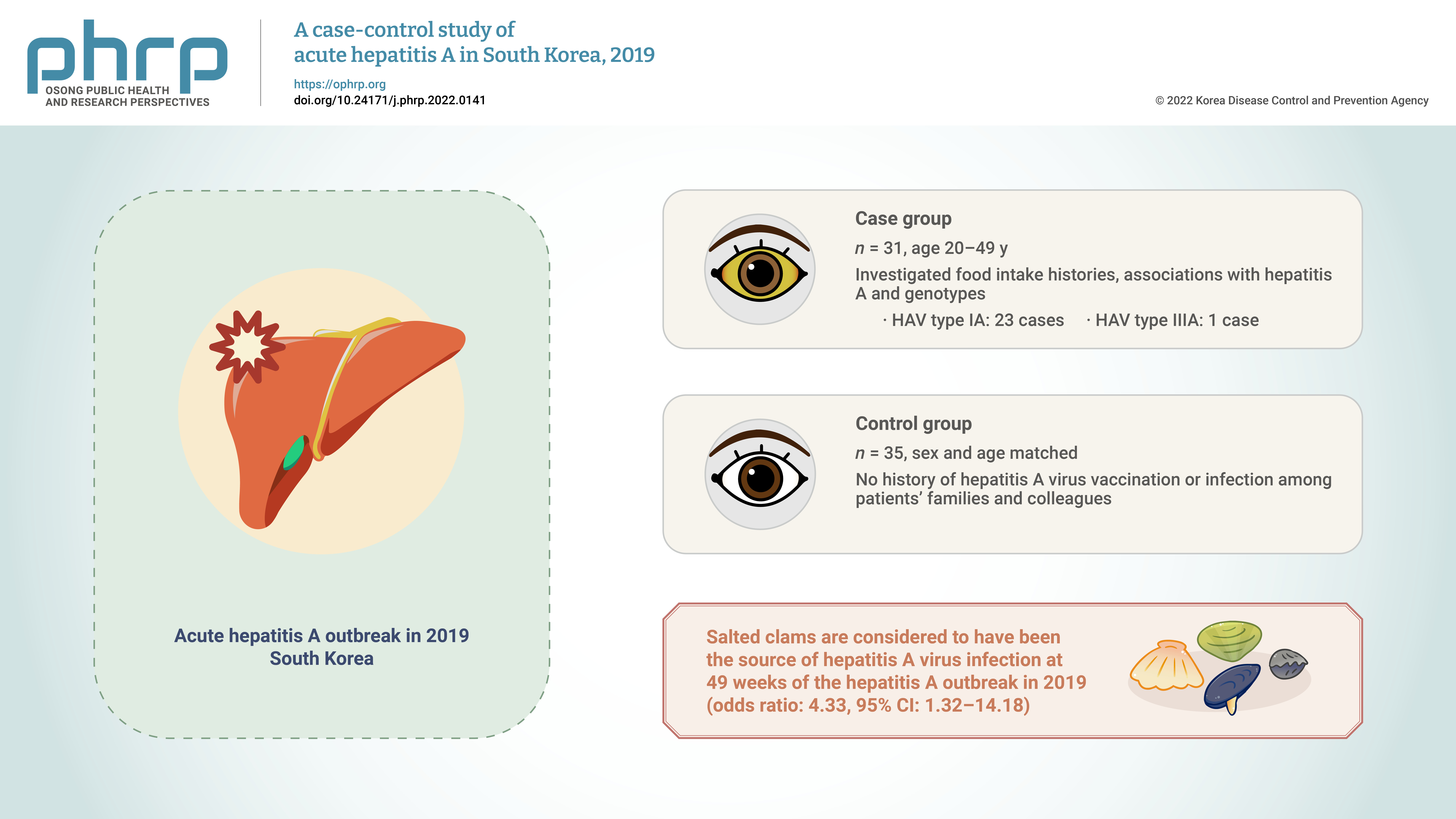
- Objectives
We aimed to reconfirm the source of hepatitis A virus (HAV) infection through epidemiological and genotype investigations of individual cases in a 2019 outbreak in South Korea. Methods: We investigated food intake histories, associations with hepatitis A, and genotypes of HAV in 31 patients with hepatitis aged 20 to 49 years registered in the integrated disease and health management system during December 1–7, 2019 (case group) and in 35 sex- and agematched people without a history of HAV vaccination or infection among patients’ families and colleagues (control group). Results: The consumption of salted clams was a significant factor (odds ratio, 4.33; 95% confidence interval, 1.32–14.18) in the risk factor analysis of food intake history. HAV genotypes were analyzed in 24 of 31 patients. Type IA and type IIIA were found in 23 and 1 cases, respectively. Conclusion: Salted clams are considered to have been the source of HAV infection at 49 weeks of the HAV outbreak in 2019; this result was consistent with that of a previous epidemiological investigation conducted by the Korea Disease Control and Prevention Agency in September 2019. Therefore, monitoring of the production and distribution of salted clams needs to be continued. -
Citations
Citations to this article as recorded by- Monitoring viruses and beta-lactam resistance genes through wastewater surveillance during a COVID-19 surge in Suwon, South Korea
Rajendra Singh, Jaewon Ryu, Sung Soo Park, Sungpyo Kim, Keugtae Kim
Science of The Total Environment.2024; 922: 171223. CrossRef - Prevalence of foodborne viruses and influenza A virus from poultry processing plants to retailed chickens
Daseul Yeo, Mengxiao Song, Md. Iqbal Hossain, Soontag Jung, Zhaoqi Wang, Dong Joo Seo, Min Suk Rhee, Changsun Choi
Frontiers in Sustainable Food Systems.2023;[Epub] CrossRef - A Study on the Detection Rate of Hepatitis A from Gastroenteritis Patients and the Genotype Analysis of Hepatitis A Virus in Busan
Sun Hee Park, Chanhee Kim, Summi Lee, Jihye Jeong, Junghye Choi, Seung Ju Lee
Journal of Bacteriology and Virology.2023; 53(2): 74. CrossRef - A Study on the Detection Rate of Hepatitis A from Gastroenteritis Patients and the Genotype Analysis of Hepatitis A Virus in Busan
Sun Hee Park, Chanhee Kim, Summi Lee, Jihye Jeong, Junghye Choi, Seung Ju Lee
Journal of Bacteriology and Virology.2023; 53(2): 74. CrossRef
- Monitoring viruses and beta-lactam resistance genes through wastewater surveillance during a COVID-19 surge in Suwon, South Korea
- Investigation of SARS-CoV-2 lineages and mutations circulating in a university-affiliated hospital in South Korea analyzed using Oxford Nanopore MinION sequencing
- Hyaekang Kim, Sung Hee Chung, Hyun Soo Kim, Han-Sung Kim, Wonkeun Song, Ki Ho Hong, Jae-Seok Kim
- Osong Public Health Res Perspect. 2022;13(5):360-369. Published online October 11, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0183
- 3,076 View
- 76 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF Supplementary Material
Supplementary Material - Objectives
Despite the introduction of vaccines, treatments, and massive diagnostic testing, the evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has continued to overcome barriers that had slowed its previous spread. As the virus evolves towards increasing fitness, it is critical to continue monitoring the occurrence of new mutations that could evade human efforts to control them. Methods: We performed whole-genome sequencing using Oxford Nanopore MinION sequencing on 58 SARS-CoV-2 isolates collected during the ongoing coronavirus disease 2019 pandemic at a tertiary hospital in South Korea and tracked the emergence of mutations responsible for massive spikes in South Korea. Results: The differences among lineages were more pronounced in the spike gene, especially in the receptor-binding domain (RBD), than in other genes. Those RBD mutations could compromise neutralization by antibodies elicited by vaccination or previous infections. We also reported multiple incidences of Omicron variants carrying mutations that could impair the diagnostic sensitivity of reverse transcription-polymerase chain reaction-based testing. Conclusion: These results provide an understanding of the temporal changes of variants and mutations that have been circulating in South Korea and their potential impacts on antigenicity, therapeutics, and diagnostic escape of the virus. We also showed that the utilization of the nanopore sequencing platform and the ARTIC workf low can provide convenient and accurate SARS-CoV-2 genomic surveillance even at a single hospital. -
Citations
Citations to this article as recorded by- Understanding large scale sequencing datasets through changes to protein folding
David Shorthouse, Harris Lister, Gemma S Freeman, Benjamin A Hall
Briefings in Functional Genomics.2024;[Epub] CrossRef - Molecular epidemiology of SARS‐CoV‐2 in Mongolia, first experience with nanopore sequencing in lower‐ and middle‐income countries setting
Munkhtuya Erendereg, Suvd Tumurbaatar, Otgonjargal Byambaa, Gerelmaa Enebish, Natsagdorj Burged, Tungalag Khurelsukh, Nomin‐Erdene Baatar, Badmaarag Munkhjin, Jargaltulga Ulziijargal, Anuujin Gantumur, Oyunbaatar Altanbayar, Ochbadrakh Batjargal, Delgermu
Immunity, Inflammation and Disease.2023;[Epub] CrossRef
- Understanding large scale sequencing datasets through changes to protein folding
- Clinical outcomes of remdesivir-treated COVID-19 patients in South Korea
- Mi Yu, Bryan Inho Kim, Jungyeon Kim, Jin Gwack
- Osong Public Health Res Perspect. 2022;13(5):370-376. Published online October 18, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0138
- 2,062 View
- 71 Download
- 2 Web of Science
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Objectives
This study analyzed the clinical outcomes of remdesivir treatment in coronavirus disease 2019 (COVID-19) patients in South Korea.
Methods
This retrospective cohort study involved the secondary analysis of epidemiological data. Among patients diagnosed with COVID-19 from July 2, 2020 to March 23, 2021 (12 AM), 4,868 who received oxygen therapy and were released from isolation after receiving remdesivir treatment were assigned to the treatment group, and 6,068 patients who received oxygen therapy but not remdesivir were assigned to the untreated group. The study subjects included children under the age of 19. The general characteristics and severity were compared between the groups. Differences in the time to death and mortality were also compared.
Results
In the untreated group, the hazard ratio [HR] for mortality was 1.59 among patients aged ≥70 years and 2.32 in patients with severe disease in comparison to the treatment group. In a comparison of survival time among patients with severe disease aged ≥70 years, the HR for mortality before 50 days was 2.09 in the untreated group compared to the treatment group.
Conclusion
Patients with remdesivir treatment showed better clinical outcomes in this study, but these results should be interpreted with caution since this study was not a fully controlled clinical trial. -
Citations
Citations to this article as recorded by- The Role of Multidimensional Prognostic Index to Identify Hospitalized Older Adults with COVID-19 Who Can Benefit from Remdesivir Treatment: An Observational, Prospective, Multicenter Study
Carlo Custodero, Nicola Veronese, Eva Topinkova, Helena Michalkova, Maria Cristina Polidori, Alberto Cella, Alfonso J. Cruz-Jentoft, Christine A. F. von Arnim, Margherita Azzini, Heidi Gruner, Alberto Castagna, Giovanni Cenderello, Romina Custureri, Tania
Drugs & Aging.2023; 40(7): 643. CrossRef - Remdesivir: A Review in COVID-19
Hannah A. Blair
Drugs.2023; 83(13): 1215. CrossRef
- The Role of Multidimensional Prognostic Index to Identify Hospitalized Older Adults with COVID-19 Who Can Benefit from Remdesivir Treatment: An Observational, Prospective, Multicenter Study
Brief Reports
- Presumed population immunity to SARS-CoV-2 in South Korea, April 2022
- Eun Jung Jang, Young June Choe, Seung Ah Choe, Yoo-Yeon Kim, Ryu Kyung Kim, Jia Kim, Do Sang Lim, Ju Hee Lee, Seonju Yi, Sangwon Lee, Young-Joon Park
- Osong Public Health Res Perspect. 2022;13(5):377-381. Published online October 14, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0209
- 2,348 View
- 71 Download
- 3 Web of Science
- 4 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF 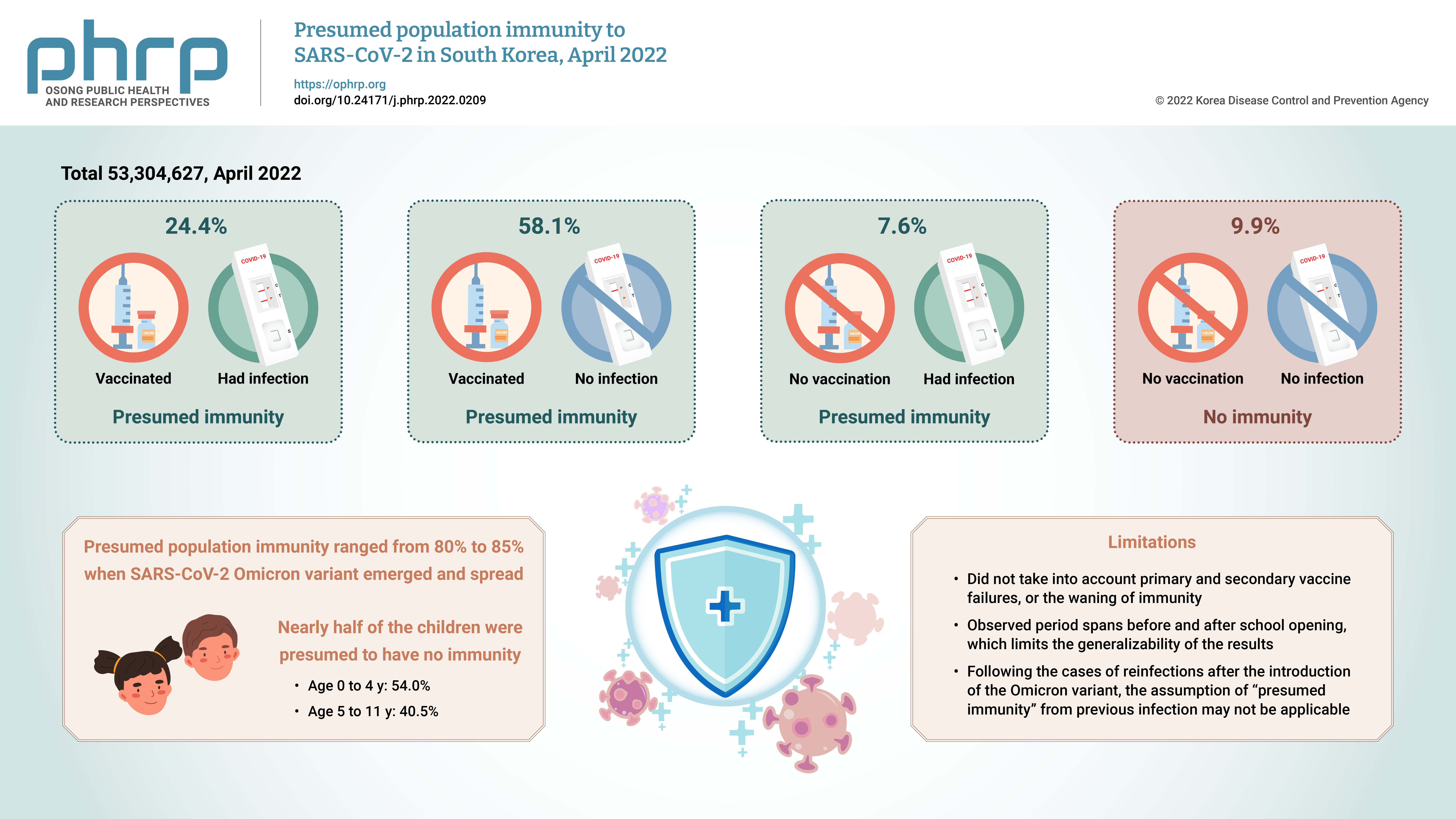
- Objectives
We estimated the overall and age-specific percentages of the Korean population with presumed immunity against severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) as of April 2022 using the national registry.
Methods
We used the national coronavirus disease 2019 (COVID-19) infection and vaccination registry from South Korea, as described to define individuals with a previous history of COVID-19 infection, vaccination, or both, as persons with presumed immunity.
Results
Of a total of 53,304,627 observed persons, 24.4% had vaccination and infection, 58.1% had vaccination and no infection, 7.6% had infection and no vaccination, and 9.9% had no immunity. The SARS-CoV-2 Omicron variant emerged at a time when the presumed population immunity ranged from 80% to 85%; however, nearly half of the children were presumed to have no immunity.
Conclusion
We report a gap in population immunity, with lower presumed protection in children than in adults. The approach presented in this work can provide valuable informed tools to assist vaccine policy-making at a national level. -
Citations
Citations to this article as recorded by- Realistic Estimation of COVID-19 Infection by Seroprevalence Surveillance of SARS-CoV-2 Antibodies: An Experience From Korea Metropolitan Area From January to May 2022
In Hwa Jeong, Jong-Hun Kim, Min-Jung Kwon, Jayoung Kim, Hee Jin Huh, Byoungguk Kim, Junewoo Lee, Jeong-hyun Nam, Eun-Suk Kang
Journal of Korean Medical Science.2024;[Epub] CrossRef - Epidemiology of Coronavirus Disease 2019 in Infants and Toddlers, Seoul, South Korea
JiWoo Sim, Euncheol Son, Young June Choe
Pediatric Infection & Vaccine.2024; 31(1): 94. CrossRef - Predicting adherence to COVID-19 preventive measures among South Korean adults aged 40 to 69 Years using the expanded health empowerment model
Su-Jung Nam, Tae-Young Pak
SSM - Population Health.2023; 22: 101411. CrossRef - Acute COVID-19 in unvaccinated children without a history of previous infection during the delta and omicron periods
Jee Min Kim, Ji Yoon Han, Seung Beom Han
Postgraduate Medicine.2023; 135(7): 727. CrossRef
- Realistic Estimation of COVID-19 Infection by Seroprevalence Surveillance of SARS-CoV-2 Antibodies: An Experience From Korea Metropolitan Area From January to May 2022
- Adverse events of the Pfizer-BioNTech COVID-19 vaccine in Korean children and adolescents aged 5 to 17 years
- Seontae Kim, Yeseul Heo, Soon-Young Seo, Do Sang Lim, Enhi Cho, Yeon-Kyeng Lee
- Osong Public Health Res Perspect. 2022;13(5):382-390. Published online October 14, 2022
- DOI: https://doi.org/10.24171/j.phrp.2022.0233
- 2,386 View
- 111 Download
- 2 Crossref
-
 Abstract
Abstract
 PDF
PDF - Objectives
This study aimed to identify potential safety signals and adverse events following the primary Pfizer-BioNTech coronavirus disease 2019 (COVID-19) vaccination series among children and adolescents aged 5 to 17 years in the Republic of Korea. Methods: Adverse events reported through the COVID-19 vaccination management system (CVMS, a web-based passive vaccine safety surveillance system) and adverse events and health conditions collected from a text message-based survey were analyzed. Results: A total of 14,786 adverse events among 5 to 17-year-old children and adolescents were reported in the CVMS; 14,334 (96.9%) were non-serious and 452 (3.1%) were serious, including 125 suspected cases of acute cardiovascular injury and 101 suspected cases of anaphylaxis. The overall reporting rate was lower in 5 to 11-year-old children (64.5 per 100,000 doses) than in 12 to 17-year-old adolescents (300.5 per 100,000 doses). The text message survey identified that local and systemic adverse events after either dose were reported less frequently in 5 to 11-year-old children than in 12 to 17-year-old adolescents (p<0.001). The most commonly reported adverse events were pain at the injection site, myalgia, headache, and fatigue/tiredness. Conclusion: The overall results are consistent with the results of controlled trials; serious adverse events were extremely rare among 5 to 17-year-old children and adolescents following Pfizer-BioNTech COVID-19 vaccination. Adverse events were less frequent in children aged 5 to 11 years than in adolescents aged 12 to 17 years. -
Citations
Citations to this article as recorded by- Safety monitoring of COVID-19 vaccines: February 26, 2021, To June 4, 2022, Republic of Korea
Yeon-Kyeng Lee, Yunhyung Kwon, Yesul Heo, Eun Kyoung Kim, Seung Yun Kim, Hoon Cho, Seontae Kim, Mijeong Ko, Dosang Lim, Soon-Young Seo, Enhi Cho
Clinical and Experimental Pediatrics.2023; 66(10): 415. CrossRef - Effectiveness of the BNT162b2 vaccine in preventing morbidity and mortality associated with COVID-19 in children aged 5 to 11 years: A systematic review and meta-analysis
Sumayyah Ebrahim, Ntombifuthi Blose, Natasha Gloeck, Ameer Hohlfeld, Yusentha Balakrishna, Rudzani Muloiwa, Andy Gray, Andy Parrish, Karen Cohen, Ruth Lancaster, Tamara Kredo, Julia Robinson
PLOS Global Public Health.2023; 3(12): e0002676. CrossRef
- Safety monitoring of COVID-19 vaccines: February 26, 2021, To June 4, 2022, Republic of Korea





 First
First Prev
Prev


