Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(4); 2023 > Article
-
Short Communication
Epidemiological characteristics of carbapenemase-producing Enterobacteriaceae outbreaks in the Republic of Korea between 2017 and 2022 -
Hyoseon Jeong
 , Junghee Hyun
, Junghee Hyun , Yeon-Kyeng Lee
, Yeon-Kyeng Lee
-
Osong Public Health and Research Perspectives 2023;14(4):312-320.
DOI: https://doi.org/10.24171/j.phrp.2023.0069
Published online: August 21, 2023
Division of Healthcare Associated Infection Control, Bureau of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agency, Cheongju, Republic of Korea
- Corresponding author: Yeon-Kyeng Lee Division of Healthcare Associated Infection Control, Bureau of Healthcare Safety and Immunization, Korea Disease Control and Prevention Agency, 187 Osongsaengmyeong 2-ro, Osong-eup, Heungdeok-gu, Cheongju 28159, Republic of Korea E-mail: yeonkyenglee@korea.kr
- Hyoseon Jeong and Junghee Hyun contributed equally to this study as co-first authors.
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Abstract
-
Objectives
- We aimed to describe the epidemiological characteristics of carbapenemase-producing Enterobacteriaceae (CPE) outbreaks in healthcare settings in the Republic of Korea between 2017 and 2022.
-
Methods
- Under the national notifiable disease surveillance system, we obtained annual descriptive statistics regarding the isolated species, carbapenemase genotype, healthcare facility type, outbreak location and duration, and number of patients affected and recommended interventions. We used epidemiological investigation reports on CPE outbreaks reported to Korea Disease Control and Prevention Agency from June 2017 to September 2022.
-
Results
- Among the 168 reports analyzed, Klebsiella pneumoniae (85.1%) was the most frequently reported species, while K. pneumoniae carbapenemase (KPC, 82.7%) was the most common carbapenemase genotype. Both categories increased from 2017 to 2022 (p<0.01). General hospitals had the highest proportion (54.8%), while tertiary general hospitals demonstrated a decreasing trend (p<0.01). The largest proportion of outbreaks occurred exclusively in intensive care units (ICUs, 44.0%), and the frequency of concurrent outbreaks in ICUs and general wards increased over time (p<0.01). The median outbreak duration rose from 43.5 days before the coronavirus disease 2019 (COVID-19) pandemic (2017–2019) to 79.5 days during the pandemic (2020–2022) (p=0.01), and the median number of patients associated with each outbreak increased from 5.0 to 6.0 (p=0.03). Frequently recommended interventions included employee education (38.1%), and 3 or more measures were proposed for 45.2% of outbreaks.
-
Conclusion
- In the Republic of Korea, CPE outbreaks have been consistently dominated by K. pneumoniae and KPC. The size of these outbreaks increased during the COVID-19 pandemic. Our findings highlight the need for continuing efforts to control CPE outbreaks using a multimodal approach, while considering their epidemiology.
- Carbapenem-resistant Enterobacteriaceae (CRE) frequently exhibit a wide spectrum of antibiotic resistance, which restricts treatment options and leads to various infections, including urinary tract infections, pneumonia, and sepsis [1,2]. Among CRE strains, carbapenemase-producing Enterobacteriaceae (CPE) can transfer resistance genes to other Gram-negative bacilli through plasmids or other mechanisms, resulting in more rapid dissemination than non-CPE isolates [3].
- A CPE strain was first identified in a healthcare facility in the Republic of Korea on December 3, 2010 [4], underscoring specific public health and clinical priorities. Consequently, all CRE cases have been classified as notifiable diseases across all healthcare facilities since June 3, 2017 [5,6]. According to data from the mandatory surveillance system of the Korea Disease Control and Prevention Agency (KDCA), the number of patients with CRE in healthcare facilities has steadily risen over time, increasing from 15,369 in 2019 to 23,311 in 2021. The percentage of CPE among patients with CRE has also demonstrated an annual upward trend, growing from 57.8% in 2019 to 63.4% in 2021 [7]. This increase aligned with a rising trend in ertapenem resistance among Klebsiella pneumoniae isolated from blood; this rate grew from 1.1% in 2019 to 6.8% in 2021, according to data from the Korean Global Antimicrobial Surveillance System [8].
- To manage CPE, which is known to significantly impact the spread of CRE [3], the KDCA collects data on all epidemiological investigations conducted by local governments (17 cities and provinces) related to CPE outbreaks [6]. Our study was designed to describe the epidemiological characteristics of CPE outbreaks in healthcare settings by analyzing the epidemiological investigation data reported to the KDCA between June 2017 and September 2022, following the transition to a mandatory surveillance system.
Introduction
- National Surveillance of CPE Outbreaks
- In the Korean Notifiable Disease Surveillance System, CRE is defined as Enterobacteriaceae resistant to at least 1 carbapenem-based antibiotic (including imipenem, meropenem, ertapenem, and doripenem), as identified in clinical samples from healthcare settings, regardless of patient symptoms. All CRE-positive samples must be tested for carbapenemase, as CPE is strictly defined as carbapenemase-producing CRE. When 2 or more patients with CPE and epidemiological links are reported at a healthcare facility, epidemiologists of the city or province are required to conduct an investigation in accordance with the Guidelines for Healthcare-Associated Infectious Diseases [6].
- Database
- We obtained data on CPE outbreaks from the KDCA mandatory surveillance system database. Epidemiological investigators from cities or provinces are required to report outbreaks at healthcare facilities to this system. Patient identification and sensitive information are not collected.
- Case Definitions
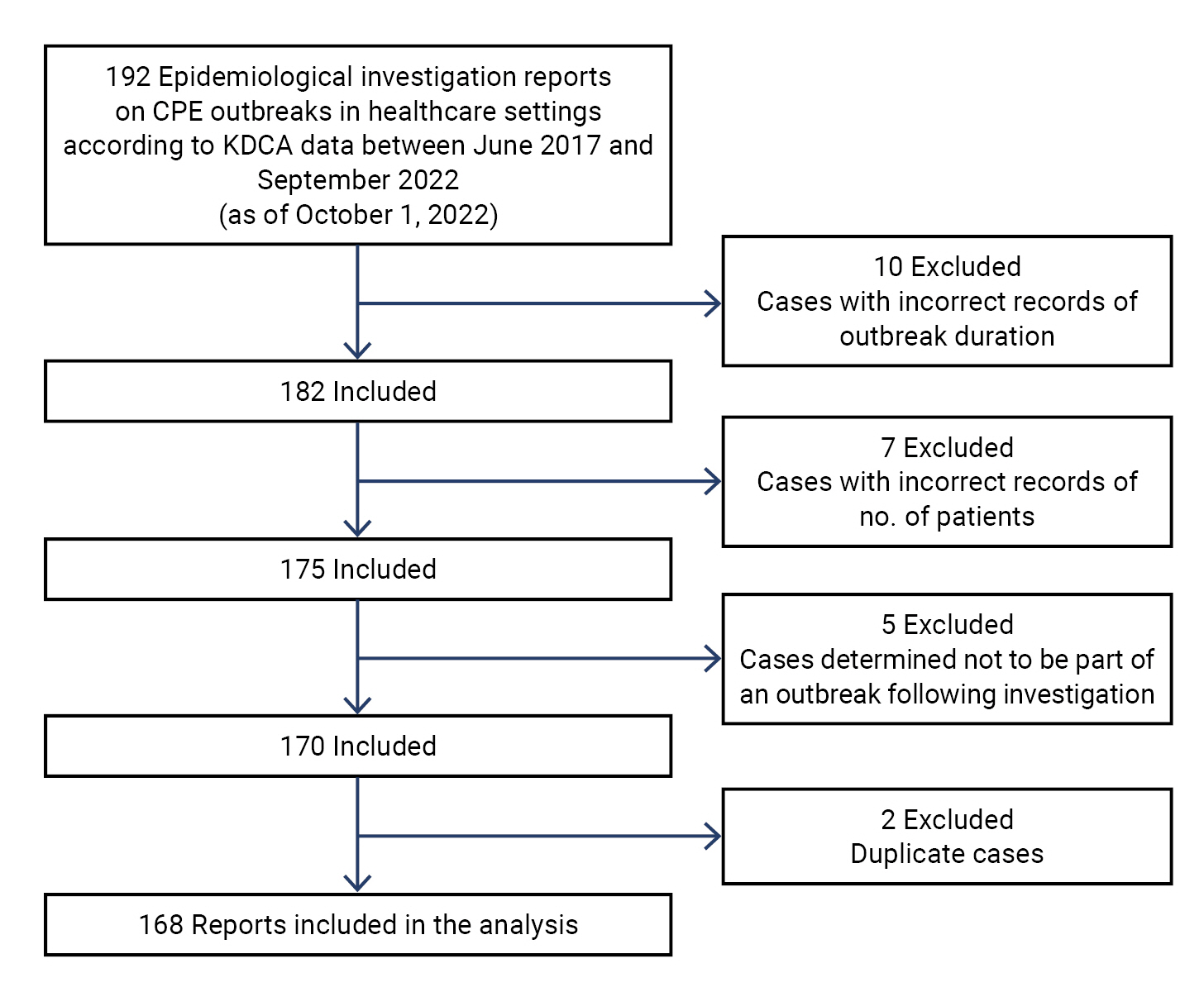
- A CPE outbreak was defined as the presence of at least 2 patients with CPE and epidemiological associations within a healthcare facility, as investigated by local epidemiologists between June 2017 and September 2022. As of October 1, 2022, the KDCA had received reports of 192 cases. From these, we excluded cases with incorrect records of outbreak duration (n=10) and number of patients (n=7), cases determined not to be part of an outbreak following epidemiological investigation (n=5), and duplicate cases (n=2). As a result, a total of 168 reports were included in the analysis (Figure 1).
- Classification of Variables
- Healthcare facilities were categorized according to the Medical Service Act as follows: tertiary general hospitals, general hospitals, hospitals, and intermediate care hospitals. General hospitals included healthcare facilities with 100 or more beds, with those possessing a high level of expertise in treating severe diseases designated as tertiary general hospitals. Hospitals comprised healthcare facilities with 30 or more beds; among these, institutions that provided care for patients with geriatric or chronic diseases, as well as patients recovering from surgery or injury, were classified as intermediate care hospitals.
- Local epidemiologists recommended intervention measures following epidemiological investigations of CPE outbreaks. In our study, these measures were categorized based on the type of infection prevention and control (IPC), consistent with the descriptive content of each report. After accounting for the IPC strategies for CPE at the facility level [3,9–11] and the current state of Korean healthcare facilities, we selected the following categories: education of employees, including caregivers; active surveillance of high-risk groups; contact precautions; environmental cleaning; screening of patients with CRE contacts; patient isolation or cohorting; support for staff and infrastructure; surveillance of environmental cultures; on-site monitoring; improvement of bed density; timely notification of patients with CRE to the KDCA; and antibiotic stewardship. Two independent researchers participated in the categorization process, and when disagreements occurred, a final decision was made through discussion.
- Statistical Analysis
- We present annual descriptive statistics on the isolated species, carbapenemase genotype, healthcare facility type, outbreak location and duration, and number of patients associated with CPE outbreaks. Additionally, we display the frequency of each recommended intervention following the epidemiological investigation, according to the type of healthcare facility. Variables are presented as either the number and percentage of outbreaks or the median and interquartile range (IQR). The Cochran-Armitage test (a yearly trend analysis adjusted for the number of outbreaks on a 12-month scale) or the Fisher exact test was utilized to compare categorical variables. For continuous variables that did not meet the normality assumption, the Mann-Whitney U-test was performed. The significance level was set at p<0.05, and R statistical software ver. 4.2.0 (The R Foundation) was employed for the statistical analysis.
- Ethics Approval
- This study received approval from the Institutional Review Board (IRB) of the KDCA (IRB No: 2023-01-02-PE-A) and was conducted in accordance with the principles of the Declaration of Helsinki.
Materials and Methods
- A total of 168 epidemiological investigations of CPE outbreaks in healthcare settings were reported to the KDCA between June 2017 and September 2022 (Table 1).
- Clinical Characteristics of CPE Outbreaks
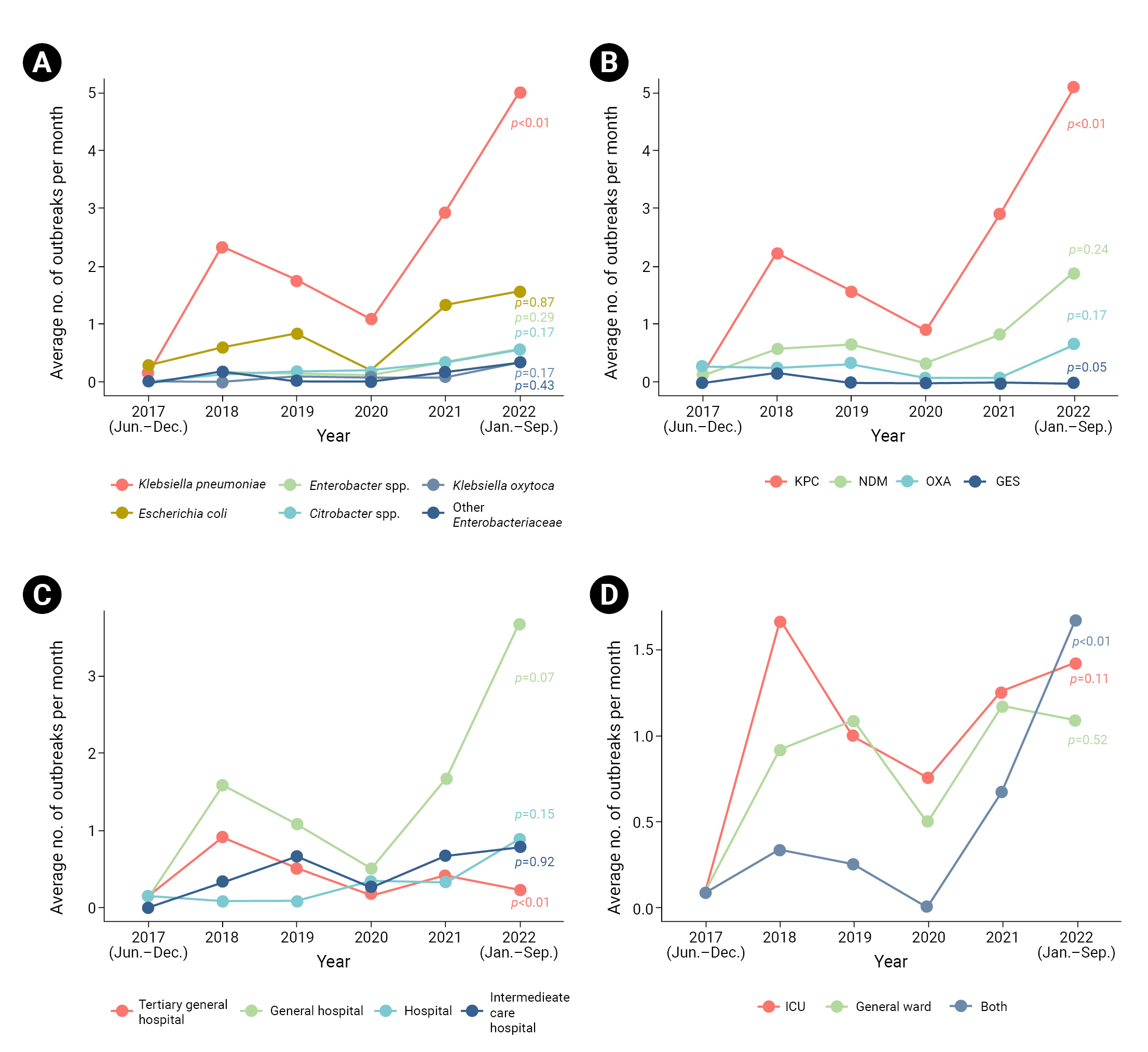
- The most frequently isolated species was K. pneumoniae (143 cases, 85.1%), followed by Escherichia coli (51 cases, 30.4%), and Enterobacter spp. and Citrobacter spp. (14 cases, 8.3% each) (Table 1). Outbreaks of K. pneumoniae significantly increased over time (p<0.01) (Figure 2). The most commonly detected carbapenemase genotype was K. pneumoniae carbapenemase (KPC), affecting 139 cases (82.7%), followed by New Delhi metallo-β-lactamase (47 cases, 28.0%) and oxacillinase (17 cases, 10.1%). The proportion of outbreaks in which KPC was detected showed an increasing trend (p<0.01).
- Epidemiological Characteristics of CPE Outbreaks
- Among the types of healthcare facilities, epidemiological investigations were implemented most frequently in general hospitals (92 cases, 54.8%) (Table 1). A declining trend was observed in tertiary general hospitals (p<0.01) (Figure 2). Within facilities, intensive care units (ICUs) were the most common location of outbreaks (74 cases, 44.0%). The frequency of outbreaks occurring simultaneously in ICUs and general wards increased significantly over time (p<0.01).
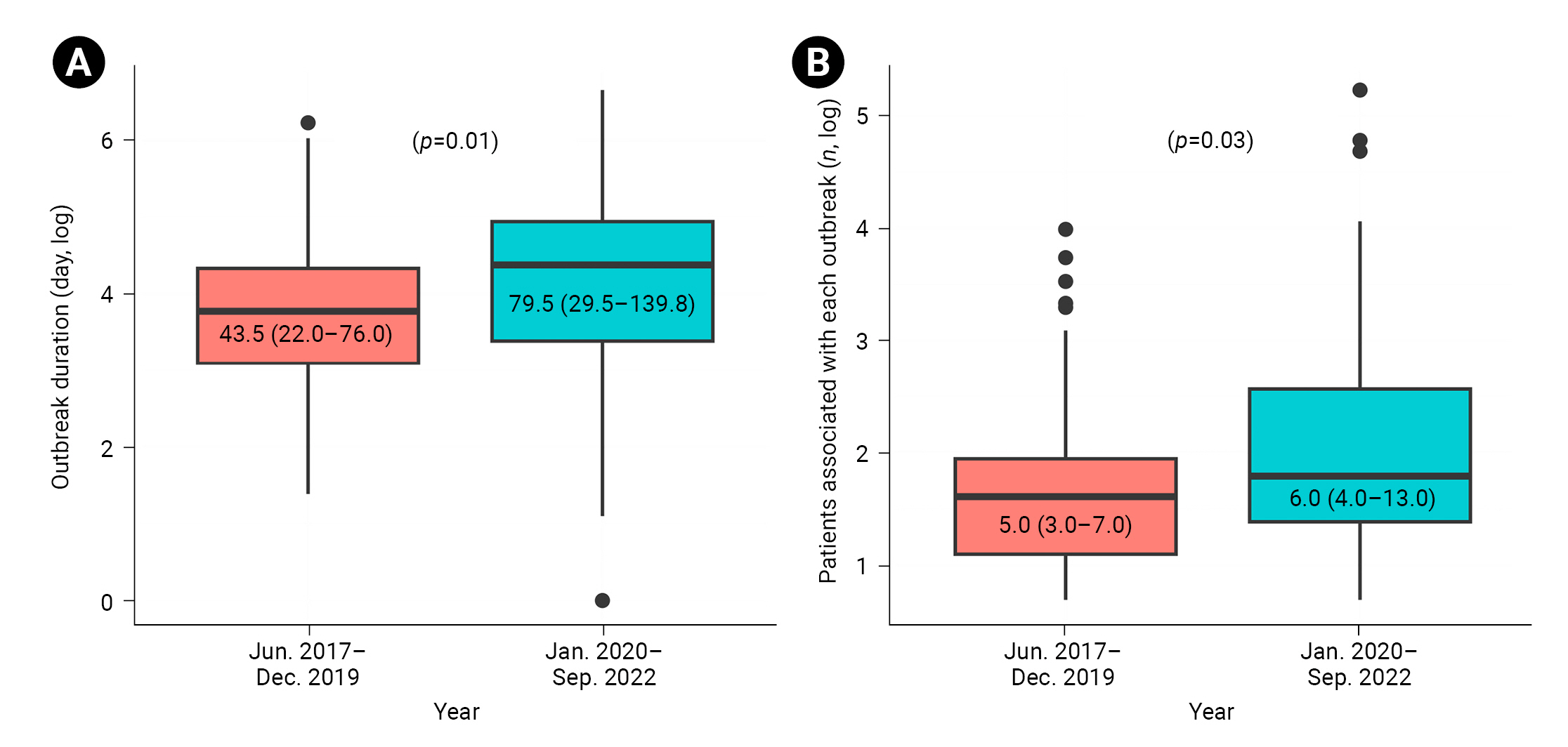
- No statistically significant variations in the duration or number of patients associated with each outbreak were observed by year. However, a significant difference emerged when we compared the periods before and during the coronavirus disease 2019 (COVID-19) pandemic (2017–2019 and 2020–2022, respectively) (Figure 3). The median outbreak duration overall was 61.0 days (IQR, 26.8–121.0), with an increase from 43.5 days (IQR, 22.0–76.0) prior to the pandemic to 79.5 days (IQR, 29.5–139.8) during the pandemic (p=0.01). The median number of patients overall was 6.0 (IQR, 3.0–11.0), with an increase from 5.0 (IQR, 3.0–7.0) before the pandemic to 6.0 (IQR, 4.0–13.0) during the pandemic (p=0.03).
- Recommended Interventions for Controlling CPE Outbreaks
- In 146 cases (86.1%), additional intervention measures were advised, while no measures were recommended in 22 cases (13.1%) (Table 2). Notably, simultaneous implementation of 3 or more interventions was suggested for 76 cases (45.2%). The most frequently recommended intervention measure was employee education (64 cases, 38.1%), followed by active surveillance of high-risk groups (32.7%), contact precautions (31.5%), and environmental cleaning (30.4%). Recommendations for environmental cleaning (p<0.01), patient isolation or cohorting (p=0.04), surveillance of environmental cultures (p=0.03), and timely notification of CRE to the KDCA (p=0.02) differed significantly depending on the type of healthcare facility.
Results
- During the early stages of the COVID-19 pandemic in 2020, the number of CPE-related epidemiological investigation reports was lower than in previous years, but it gradually increased over time. A report from the European Centre for Disease Prevention and Control also indicated a reduced number of E. coli cases reported in 2020, which may be attributed to a decrease in healthcare activities, such as the reporting of antibiotic resistance rates, due to the COVID-19 pandemic [12].
- Among the CPE outbreaks analyzed in the present study, K. pneumoniae and KPC showed increasing trends over time and were the most commonly detected species and carbapenemase genotype, respectively (with both found in ≥80% of outbreaks). This is consistent with other Korean studies [7,13]. The United States Centers for Disease Control and Prevention recommended strengthening contact tracing and surveillance in healthcare facilities to detect relatively rare carbapenemases, such as New Delhi metallo-β-lactamase [14]. Considering the rising trend of K. pneumoniae and KPC dominance within outbreaks, it may be necessary to enhance epidemiological investigations and IPC measures to control outbreaks associated with relatively rare species or carbapenemase types.
- In the present study, more than 50% of epidemiological investigations were conducted in general hospitals; additionally, outbreaks in tertiary general hospitals investigated by local epidemiologists demonstrated a sharp decline over time. A previous study of patients with CRE, utilizing KDCA data from 2021, revealed that 42.0% of cases occurred in general hospitals and 40.5% in tertiary general hospitals [7]. This finding suggests the potential for tertiary general hospitals to carry out independent epidemiological investigations and IPC measures, provided they possess adequate staffing levels and expertise.
- Regarding outbreak location within healthcare facilities, over 40% of outbreaks occurred exclusively within ICUs. A history of ICU admission is generally regarded as a risk factor for CRE [9,15]. In a study by Kim et al. [13] conducted in an ICU, pneumonia and chronic respiratory disease, previous antibiotic use, and history of nasogastric tube placement were identified as risk factors for CPE. This suggests the need for more proactive IPC measures in high-risk groups, with a focus on ICUs during CPE outbreaks. The recent increase in outbreaks occurring simultaneously in ICUs and general wards underscores the importance of IPC measures and ward separation to limit further transmission.
- The duration and number of patients associated with each outbreak were significantly greater during the COVID-19 pandemic compared to the pre-pandemic period. This may be due to a decrease in non-urgent healthcare activities not directly related to COVID-19 [12,16]. For instance, regular active surveillance of high-risk groups for CPE might have been limited, as personnel and resources were redirected towards contact tracing, laboratory testing, and isolation of COVID-19 patients. Given the need for rapid CPE testing [17], early implementation of epidemiological investigations and IPC measures is also crucial to block further transmission, as well as to avoid prolonging the outbreak and incurring increased disease burden.
- The World Health Organization recommends implementing multimodal strategies (consisting of at least 3 and usually 5 elements) across healthcare facilities for the prevention and management of the spread of carbapenem-resistant organisms [11]. Similarly, in the present study, we found that multimodal interventional approaches were concurrently advised following ≥40% of epidemiological investigations of CPE outbreaks in the Republic of Korea, regardless of the type of healthcare facility.
- Overall, employee education was the most heavily emphasized measure, along with education for caregivers who frequently interact with patients. For hospitals, environmental cleaning was most commonly recommended; however, no guidance has been made available on monitoring environmental cultures, likely due to specific facility-related circumstances. Interventions to control CPE outbreaks should be tailored to each facility’s unique situation, considering available resources and trained staff. In contrast, national guidelines in other countries suggest that acute care hospitals, rather than post-acute care hospitals, should strengthen IPC measures for patients with CRE [3,18]. For effective control of CPE outbreaks, facility-level regulations are crucial, including the implementation of basic IPC measures and outbreak investigation. These regulations should be incorporated into the education and job functions of all employees. Additionally, individual risk factors for CRE should be considered, such as antibiotic use, invasive procedures, and the use of ventilators or urethral catheters [9,19].
- Our study had some limitations. The data collected from epidemiological investigation reports may deviate from actual results due to memory bias. Additionally, some CPE-related epidemiological investigations conducted by local governments may not have been reported to the KDCA. Furthermore, we lacked detailed data on the source of infection, antibiotic use, and clinical outcomes, preventing a more in-depth analysis of outbreak-related epidemiological characteristics. However, our study presents a general overview of CPE outbreaks throughout the Republic of Korea since CRE was indicated as a mandatory notifiable disease in this country. Future studies involving detailed data should be conducted to analyze the characteristics of outbreaks responsible for differences in their spread and to consider the effectiveness of combinations of interventions to control CPE transmission.
Discussion
- We examined the epidemiological features of CPE outbreaks using data from investigations reported to the KDCA, which offered national-level information. Our results indicate that persistent efforts are necessary to manage CPE outbreaks in consideration of their epidemiology.
Conclusion
- • In the Republic of Korea, carbapenemase-producing Enterobacteriaceae outbreaks in healthcare settings were dominated consistently by Klebsiella pneumoniae and K. pneumoniae carbapenemase from 2017–2022.
- • Duration and number of patients related to each outbreak significantly increased during the coronavirus disease 2019 pandemic (2020–2022) compared with that in the pre-pandemic period (2017–2019).
- • Multimodal approaches were simultaneously recommended for interventions following a number of epidemiological investigations of carbapenemase-producing Enterobacteriaceae outbreaks.
HIGHLIGHTS
-
Ethics Approval
This study received approval from the Institutional Review Board (IRB) of the KDCA (IRB No: 2023-01-02-PE-A) and was conducted in accordance with the principles of the Declaration of Helsinki.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but can be obtained from the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: HJ, JH, YKL; Data curation: HJ, JH; Formal analysis: HJ; Methodology: HJ, JH, YKL; Project administration: HJ; Resources: HJ, JH, YKL; Supervision: YKL; Validation: HJ, JH, YKL; Visualization: HJ, YKL; Writing–original draft: HJ, JH; Writing–review & editing: HJ, JH, YKL. All authors read and approved the final manuscript.
Article information


| Characteristic | Total (n=168) | 2017 (Jun.–Dec.) (n=3) | 2018 (n=35) | 2019 (n=28) | 2020 (n=15) | 2021 (n=37) | 2022 (Jan.–Sep.) (n=50) |
|---|---|---|---|---|---|---|---|
| Isolated species | |||||||
| Klebsiella pneumoniae | 143 (85.1) | 1 (33.3) | 28 (80.0) | 21 (75.0) | 13 (86.7) | 35 (94.6) | 45 (90.0) |
| Escherichia coli | 51 (30.4) | 2 (66.7) | 7 (20.0) | 10 (35.7) | 2 (13.3) | 16 (43.2) | 14 (28.0) |
| Enterobacter spp. | 14 (8.3) | 0 (0) | 2 (5.7) | 2 (7.1) | 1 (6.7) | 4 (10.8) | 5 (10.0) |
| Citrobacter spp. | 14 (8.3) | 0 (0) | 1 (2.9) | 2 (7.1) | 2 (13.3) | 4 (10.8) | 5 (10.0) |
| Klebsiella oxytoca | 6 (3.6) | 0 (0) | 0 (0) | 1 (3.6) | 1 (6.7) | 1 (2.7) | 3 (6.0) |
| Other Enterobacteriaceae spp. | 7 (4.2) | 0 (0) | 2 (5.7) | 0 (0) | 0 (0) | 2 (5.4) | 3 (6.0) |
| Carbapenemase genotype | |||||||
| KPC | 139 (82.7) | 1 (33.3) | 27 (77.1) | 19 (67.9) | 11 (73.3) | 35 (94.6) | 46 (92.0) |
| NDM | 47 (28.0) | 1 (33.3) | 7 (20.0) | 8 (28.6) | 4 (26.7) | 10 (27.0) | 17 (34.0) |
| OXA | 17 (10.1) | 2 (66.7) | 3 (8.6) | 4 (14.3) | 1 (6.7) | 1 (2.7) | 6 (12.0) |
| GES | 2 (1.2) | 0 (0) | 2 (5.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Healthcare facility type | |||||||
| Tertiary general hospital | 27 (16.1) | 1 (33.3) | 11 (31.4) | 6 (21.4) | 2 (13.3) | 5 (13.5) | 2 (4.0) |
| General hospital | 92 (54.8) | 1 (33.3) | 19 (54.3) | 13 (46.4) | 6 (40.0) | 20 (54.1) | 33 (66.0) |
| Hospital | 19 (11.3) | 1 (33.3) | 1 (2.9) | 1 (3.6) | 4 (26.7) | 4 (10.8) | 8 (16.0) |
| Intermediate care hospital | 30 (17.9) | 0 (0) | 4 (11.4) | 8 (28.6) | 3 (20.0) | 8 (21.6) | 7 (14.0) |
| Outbreak location | |||||||
| ICU | 74 (44.0) | 1 (33.3) | 20 (57.1) | 12 (42.9) | 9 (60.0) | 15 (40.5) | 17 (34.0) |
| General ward | 58 (34.5) | 1 (33.3) | 11 (31.4) | 13 (46.4) | 6 (40.0) | 14 (37.8) | 13 (26.0) |
| Botha) | 36 (21.4) | 1 (33.3) | 4 (11.4) | 3 (10.7) | 0 (0) | 8 (21.6) | 20 (40.0) |
| Outbreak duration (d) | 61.0 (26.8–121.0) | 62.0 (52.5–62.5) | 49.0 (22.0–77.5) | 35.0 (19.5–80.0) | 84.0 (23.0–154.0) | 60.0 (34.0–121.0) | 83.0 (29.5–162.2) |
| Patients related to each outbreak (n) | 6.0 (3.0–11.0) | 7.0 (4.5–13.5) | 6.0 (4.0–8.0) | 4.0 (2.8–6.0) | 5.0 (3.5–10.5) | 6.0 (3.0–11.0) | 7.0 (4.3–18.0) |
Data are presented as the no. of outbreaks (%) (multiple selection available) or median (interquartile range).
CPE, carbapenemase-producing Enterobacteriaceae; KDCA, Korea Disease Control and Prevention Agency; KPC, Klebsiella pneumoniae carbapenemase; NDM, New Delhi metallo-β-lactamase; OXA, oxacillinase; GES, Guiana extended-spectrum β-lactamase; ICU, intensive care unit.
a) Outbreaks occurred simultaneously in ICUs and general wards.
| Characteristic | Total (n=168) | Tertiary general hospital (n=27) | General hospital (n=92) | Hospital (n=19) | Intermediate care hospital (n=30) | pa) |
|---|---|---|---|---|---|---|
| Type of recommended interventions | ||||||
| Education of employees, including caregivers | 64 (38.1) | 12 (44.4) | 34 (37.0) | 10 (52.6) | 8 (26.7) | 0.28 |
| Active surveillance of high-risk groups | 55 (32.7) | 11 (40.7) | 34 (37.0) | 3 (15.8) | 7 (23.3) | 0.17 |
| Contact precautions | 53 (31.5) | 11 (40.7) | 27 (29.3) | 7 (36.8) | 8 (26.7) | 0.60 |
| Environmental cleaning | 51 (30.4) | 5 (18.5) | 23 (25.0) | 13 (68.4) | 10 (33.3) | <0.01 |
| Screening contacts of patients with CRE | 49 (29.2) | 5 (18.5) | 30 (32.6) | 7 (36.8) | 7 (23.3) | 0.40 |
| Patient isolation or cohorting | 40 (23.8) | 9 (33.3) | 16 (17.4) | 3 (15.8) | 12 (40.0) | 0.04 |
| Support for staff and infrastructure | 22 (13.1) | 2 (7.4) | 12 (13.0) | 4 (21.1) | 4 (13.3) | 0.60 |
| Surveillance of environmental cultures | 20 (11.9) | 2 (7.4) | 10 (10.9) | 0 (0) | 8 (26.7) | 0.03 |
| On-site monitoring | 15 (8.9) | 3 (11.1) | 6 (6.5) | 4 (21.1) | 2 (6.7) | 0.21 |
| Improvement of bed density | 13 (7.7) | 2 (7.4) | 7 (7.6) | 3 (15.8) | 1 (3.3) | 0.48 |
| Timely notification of CRE to KDCA | 6 (3.6) | 1 (3.7) | 1 (1.1) | 0 (0) | 4 (13.3) | 0.02 |
| Antibiotic stewardship | 4 (2.4) | 0 (0) | 4 (4.3) | 0 (0) | 0 (0) | 0.67 |
| No. of intervention types recommended for each outbreak | ||||||
| 0 (None of the above) | 22 (13.1) | 3 (11.1) | 15 (16.3) | 1 (5.3) | 3 (10.0) | 0.63 |
| 1–2 | 70 (41.7) | 12 (44.4) | 39 (42.4) | 8 (42.1) | 11 (36.7) | 0.94 |
| 3–7 | 76 (45.2) | 12 (44.4) | 38 (41.3) | 10 (52.6) | 16 (53.3) | 0.61 |
- 1. van Duin D, Doi Y. The global epidemiology of carbapenemase-producing Enterobacteriaceae. Virulence 2017;8:460−9.ArticlePubMedPMC
- 2. Tilahun M, Kassa Y, Gedefie A, et al. Emerging carbapenem-resistant Enterobacteriaceae infection, its epidemiology and novel treatment options: a review. Infect Drug Resist 2021;14:4363−74.ArticlePubMedPMCPDF
- 3. National Center for Emerging and Zoonotic Infectious Diseases (U.S.), Division of Healthcare Quality Promotion. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE): November 2015 update-CRE toolkit [Internet]. National Center for Emerging and Zoonotic Infectious Diseases (U.S.); 2015 [cited 2023 May 31]. Available from: https://stacks.cdc.gov/view/cdc/79104.
- 4. Korea Disease Control and Prevention Agency (KDCA). First cases of NDM-1 (New Delhi Metallo-beta-lactamase) producing carbapenem resistant Enterobacteriaceae in Korea. KDCA; 2010. Korean.
- 5. Lee E, Lee S, Bahk H, et al. Analysis of carbapenemase-producing Enterobacteriaceae (CPE) surveillance results for 2017 in Korea: comparison with the surveillance results of the previous 5 years (2012-2016). Public Health Wkly Rep 2018;11:1586−94. Korean.
- 6. Korea Disease Control and Prevention Agency (KDCA). Guidelines for healthcare-associated infectious diseases, 2022. KDCA; 2022. Korean.
- 7. Jeong H, Hyun J, Lee Y. Characteristics of carbapenem-resistant Enterobacteriaceae (CRE) in the Republic of Korea, 2021. Public Health Wkly Rep 2022;15:2354−63. Korean.
- 8. Korea Disease Control and Prevention Agency (KDCA). National antimicrobial resistance surveillance in Korea 2021. KDCA; 2022. Korean.
- 9. van Loon K, Voor In 't Holt AF, Vos MC. A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2018;62:e01730−17.PubMed
- 10. Tomczyk S, Zanichelli V, Grayson ML, et al. Control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa in healthcare facilities: a systematic review and reanalysis of quasi-experimental studies. Clin Infect Dis 2019;68:873−84.ArticlePubMedPMC
- 11. World Health Organization (WHO). Implementation manual to prevent and control the spread of carbapenem-resistant organisms at the national and health care facility level: interim practical manual supporting implementation of the Guidelines for the prevention and control of carbapenem-resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in health care facilities. WHO; 2019.
- 12. WHO Regional Office for Europe/European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2022-2020 data. WHO Regional Office for Europe; 2022.
- 13. Kim YA, Lee SJ, Park YS, et al. Risk factors for carbapenemase-producing Enterobacterales infection or colonization in a Korean intensive care unit: a case-control study. Antibiotics (Basel) 2020;9:680. ArticlePubMedPMC
- 14. Centers for Disease Control and Prevention (CDC). Interim guidance for a public health response to contain novel or targeted multidrug-resistant organisms (MDROs). CDC; 2019.
- 15. Lee Y, Kang JE, Ham JY, et al. Risk factors of carbapenem-resistant Enterobacteriaceae acquisition at a community-based hospital. Korean J Clin Pharm 2020;30:120−6. Korean.Article
- 16. Wielders CC, Schouls LM, Woudt SH, et al. Epidemiology of carbapenem-resistant and carbapenemase-producing Enterobacterales in the Netherlands 2017-2019. Antimicrob Resist Infect Control 2022;11:57. PubMedPMC
- 17. Ahn K, Hwang GY, Kim YK, et al. Nosocomial outbreak caused by NDM-5 and OXA-181 carbapenemase co-producing Escherichia coli. Infect Chemother 2019;51:177−82.ArticlePubMedPMCPDF
- 18. Solter E, Adler A, Rubinovitch B, et al. Israeli national policy for carbapenem-resistant Enterobacteriaceae screening, carrier isolation and discontinuation of isolation. Infect Control Hosp Epidemiol 2018;39:85−9.ArticlePubMed
- 19. Nicolas-Chanoine MH, Vigan M, Laouenan C, et al. Risk factors for carbapenem-resistant Enterobacteriaceae infections: a French case-control-control study. Eur J Clin Microbiol Infect Dis 2019;38:383−93.ArticlePubMedPDF
References
Figure & Data
References
Citations

- Comparison of clinical outcomes of patients with serial negative surveillance cultures according to a subsequent polymerase chain reaction test for carbapenemase-producing Enterobacterales
H. Seo, S. Kim, Y.W. Lee, H.S. Oh, H-S. Kim, Y.K. Kim
Journal of Hospital Infection.2024; 146: 93. CrossRef - Identifying Contact Time Required for Secondary Transmission of Clostridioides difficile Infections by Using Real-Time Locating System
Min Hyung Kim, Jaewoong Kim, Heejin Ra, Sooyeon Jeong, Yoon Soo Park, Dongju Won, Hyukmin Lee, Heejung Kim
Emerging Infectious Diseases.2024;[Epub] CrossRef
- Figure
- Related articles
-
- Vaccine effectiveness and the epidemiological characteristics of a COVID-19 outbreak in a tertiary hospital in Republic of Korea
- The incidence and clinical characteristics of myocarditis and pericarditis following mRNA-based COVID-19 vaccination in Republic of Korea adolescents from July 2021 to September 2022
- Epidemiological characteristics of carbapenem-resistant Enterobacteriaceae and carbapenem-resistant Acinetobacter baumannii in a tertiary referral hospital in Korea
- Epidemiological characteristics of varicella outbreaks in the Republic of Korea, 2016–2020



 Cite
Cite