Articles
- Page Path
- HOME > Osong Public Health Res Perspect > Volume 14(5); 2023 > Article
-
Original Article
Estimating the prevalence of oral manifestations in COVID-19 patients: a systematic review -
Ankita Gupta1
 , Kriti Shrivastav2
, Kriti Shrivastav2 , Amit Agrawal3
, Amit Agrawal3 , Abhishek Purohit4
, Abhishek Purohit4 , Roshan Chanchlani5
, Roshan Chanchlani5
-
Osong Public Health and Research Perspectives 2023;14(5):388-417.
DOI: https://doi.org/10.24171/j.phrp.2023.0033
Published online: September 19, 2023
1Department of Public Health Dentistry, Rishiraj College of Dental Sciences, Bhopal, India
2Department of Oral Medicine and Radiology, Rishiraj College of Dental Sciences, Bhopal, India
3Department of Pediatrics, Gandhi Medical College and Hamidia Hospital, Bhopal, India
4Department of Dentistry, Regional Training Center for Oral Health Promotion, All India Institute of Medical Sciences, Bhopal, India
5Department of Pediatric Surgery, All India Institute of Medical Sciences, Bhopal, India
- Corresponding author: Ankita Gupta Department of Public Health Dentistry, Rishiraj College of Dental Sciences, Bhopal, MP 462036, India E-mail: ankita26gupta88@gmail.com
- Co-Corresponding author: Abhishek Purohit Department of Dentistry, Regional Training Center for Oral Health Promotion, All India Institute of Medical Sciences, Bhopal, MP 462020, India E-mail: abhishekpurohit3@gmail.com
© 2023 Korea Disease Control and Prevention Agency.
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
- 2,819 Views
- 94 Download
Abstract
-
Objectives
- Patients with coronavirus disease 2019 (COVID-19) present with a variety of oral manifestations. Therefore, we conducted a systematic review to estimate the prevalence of oral lesions among COVID-19 patients.
-
Methods
- An extensive literature search of several electronic bibliographic databases (PubMed, Scopus, Science Direct, Litcovid) was conducted to retrieve all articles published in the English language from January 1, 2020 to March 31, 2023 that reported the prevalence of oral manifestations among COVID-19 patients. A meta-analysis of pooled prevalence was performed using Jamovi ver. 2.3 (2022). The I2 and Q statistics were used to assess heterogeneity between studies, and p-values <0.01 were considered statistically significant.
-
Results
- In total, 79 studies with data from 13,252 patients were included. The articles were predominantly published in 2020 (n=33), and Italy was the most common country (n=14). Most of the affected patients more than 50 years old and women (56.6%). The most common sites of involvement were the tongue (n=65), followed by the oral mucosa (n=37) and lips (n=19). High heterogeneity was found between studies. The most common oral manifestation was taste alteration, followed by xerostomia and ulceration, showing pooled prevalence rates of 48%, 35%, and 21%, respectively.
-
Conclusion
- COVID-19 patients show various oral manifestations that may help clinicians identify the disease promptly. Recognition of the signs and symptoms of COVID-19 is critical for an early diagnosis and better prognosis.
- The novel coronavirus disease 2019 (COVID-19) has rapidly evolved into a global crisis, posing a significant challenge to public health due to its swift spread and high mortality rate. Initially identified in December 2019 in China’s Hubei Province, the disease quickly spread across the globe. By March 2020, the World Health Organization (WHO) had declared it a ‘pandemic emergency’. As of April 2023, the outbreak has resulted in over 762,201,169 confirmed cases and 6,893,190 deaths worldwide [1]. The disease’s incubation period spans from 1 to 14 days, with the most frequently observed symptoms being fever, cough, shortness of breath or difficulty breathing, and fatigue. Other less common symptoms, such as headache, loss of taste or smell, sore throat, diarrhea, and nausea or vomiting, may also manifest [2]. The severity of these symptoms can vary greatly among individuals, as it is influenced by factors such as the timing of exposure to the virus, the patient’s age and gender, and any pre-existing health conditions.
- Research has shown that the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infiltrates human cells using receptors known as angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (also referred to as transmembrane serine protease or TMPRSS2) [3]. Of these 2, the ACE2 receptor is primarily located in the cells of the lungs, liver, kidneys, and gastrointestinal (GI) tract, as well as the salivary glands and the dorsum of the tongue within the oral cavity [4]. These cells, equipped with the receptors, serve as host cells for the virus. The virus penetrates these cells and triggers an inflammatory response in the affected organs [4].
- Previously, COVID-19 was not thought to have oral symptoms, unlike other viral rash. However, the subsequent detection of SARS-CoV-2 in patients’ saliva suggested that oral manifestations could indeed be clinical characteristics of the disease [3]. The presence of the ACE2 receptor in specific oral organs, such as the tongue and salivary glands, further supports the potential involvement of the oral cavity in COVID-19 infection [3]. The prevalence of oral manifestations among COVID-19 patients is currently unknown, but several studies have attempted to determine their incidence and prevalence [5−10]. A large-scale study by Nuno-Gonzalez et al. [5] involving 666 patients found oral cavity findings in 25.65% of cases. The most frequently observed oral symptoms, as reported in a case series by Sinadinos and Shelswell [6], were blisters, ulcerations, and desquamative gingivitis. Within the oral cavity, the palate and tongue are the sites most commonly affected by COVID-19, followed by the gums and lips [7]. On the tongue, ulcerations are particularly common, especially on the dorsal surface or sides. However, only 15% of patients develop ulcerations on the ventral surface. Other possible tongue symptoms include multiple pinpoint yellowish ulcers and white plaque [3]. The presence of white plaque on the tongue’s dorsal surface is often due to fungal infections, another common oral manifestation of SARS-CoV-2, likely resulting from reduced immunity. Dima et al. [8] reported a case of a neonate with COVID-19 who developed oral cavity candidiasis. These oral symptoms are often painful, with 75% of patients reporting discomfort [7]. In another study, 25% of patients reported taste impairment, 15% experienced burning sensations, and 20% had difficulty swallowing. Taste disorders were observed in 24% of patients (ageusia), 35% (hypogeusia), and 38% (dysgeusia). These taste disorders were more prevalent in women than in men [9].
- It is crucial for dentists to understand the oral manifestations of COVID-19, as this knowledge aids in early disease diagnosis and consequently, prevents transmission. This systematic review aims to summarize the findings from existing literature on the oral manifestations of COVID-19, highlighting the role of the dentist in mitigating the severity of this deadly pandemic.
Introduction
- A systematic assessment and description of the currently reported cases and studies related to oral manifestations associated with SARS-CoV-2 infection was conducted. This review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [10].
- Eligibility Criteria
- We conducted a search for clinical evidence in the form of original, peer-reviewed journal articles. These included observational and cross-sectional studies that investigated the prevalence of oral disorders in patients with COVID-19. In addition to these, we also incorporated case reports and case series into our systematic review. The data publication range was restricted from January 1, 2020 to June 30, 2022. We further updated our research on March 31, 2023 across these databases. We did not utilize conference papers, book reviews, book chapters, letters to the editor and replies, newspaper and newsletter articles, expert opinions, or theses and dissertations. Any articles not published in English were also excluded.
- Data Sources and Search Strategy
- We carried out a comprehensive search of various electronic bibliographic databases, including PubMed, Scopus, Science Direct, and Litcovid. We gathered all articles published between January 1, 2020, and March 31, 2023. We then screened observational cross-sectional, case-control and cohort studies, case reports/series that reported on multisystem inflammatory syndrome in children, as well as letters to the editor. The 2 independent reviewers (A.G. and K.S.) conducted an electronic search of all cross-sectional studies, case reports, and case series up until March 31, 2023. They used a combination of relevant keywords, appropriately linked by Boolean operators. (1) COVID-19 OR SARS-CoV-2 OR Coronavirus disease 2019 OR novel coronavirus; (2) AND oral manifestations OR oral involvement OR oral lesions; (3) AND cross-sectional studies OR case reports OR case series.
- Selection Process
- The inclusion of studies was done in 2 phases. During the first phase, the titles of all studies were initially screened, followed by a review of their abstracts using the established inclusion and exclusion criteria. Two authors (A.G. and K.S.) independently performed this screening. If a title and abstract appeared to meet the criteria, the full article was then read and assessed for eligibility by these same 2 authors (A.G. and K.S.). Any disagreements between the authors were resolved through discussion and consensus, with the involvement of a third author (A.A.) if necessary. Duplicates were removed and irrelevant articles were excluded from the systematic review. We obtained and evaluated the full-text articles of all potentially relevant studies. In the second phase, we screened the references of all the included studies, case reports, and case series once more to identify any additional potentially eligible studies.
- Data Collection
- Three authors (A.G., K.S., and A.P.) independently extracted data from the eligible studies. In the event of disagreements, a 4th author (A.A.) was included to facilitate consensus through discussion. We included all studies that reported orofacial manifestations in patients with COVID-19. To systematically review these studies, we assessed the included studies based on demographic details such as author, year, country, study type, sample size, gender, age, study duration, medical history, intensive care unit (ICU) admission, and disease severity. In addition, we recorded details related to oral manifestations, including the affected site, onset of orofacial manifestations, general symptoms, any special investigations conducted, treatment of oral lesions, and disease outcome. The flow diagram for article inclusion is depicted in Figure 1.
- Assessment of Risk of Bias of the Included Studies
- The Joanna Briggs Institute (JBI) Critical Appraisal Tools for use in systematic reviews of cross-sectional studies, case-control studies, case reports, and case series were used to assess the risk of bias and the individual quality of the selected studies [11]. Each type of study was assessed using its respective checklist, with each question offering 3 possible responses: yes, no, or unclear. Two blinded reviewers (A.G. and K.S.) evaluated the risk of bias in each study, using a scoring system agreed upon by all reviewers. Following the assessment, studies were categorized based on their scores: high bias (if the study scored up to 49% “yes”), moderate bias (if the study scored between 50% and 69% “yes”), and low bias (if the study scored more than 70% “yes”).
- Statistical Analysis
- Qualitative data were reorganized by grouping and comparing the information reported in the studies. Conditions affecting the oral and mucosal areas were summarized using schematic diagrams. The primary outcome of interest was the prevalence of oral symptoms in COVID-19 patients. The prevalence of oral lesions was categorized into subgroups such as taste alteration, red and white lesions, vesiculobullous lesions, xerostomia, ulceration, burning sensation, and salivary gland disorders. A meta-analysis of the combined prevalence was then conducted using Jamovi ver. 2.3 (2022; https://www.jamovi.org). To evaluate heterogeneity between studies, the I2 and Q statistics were utilized, with p-values less than 0.01 considered statistically significant. The analyses were conducted using a random model. Oral lesions in COVID-19 patients, as reported in case reports and case series, were not included in the meta-analysis.
Materials and Methods
- After conducting the initial search, a total of 275 articles were found. Of these, 79 articles were selected for inclusion in the final analysis [5,6,8,9,12−86].
- Characteristics of the Studies
- The demographic characteristics of the populations in the studies included in our analysis (n=79) are detailed in Table 1. We extracted data from these 79 studies [5,6,8,9,12−86], which encompassed a total of 13,252 patients. The individual sample sizes within these studies varied, ranging from as few as 14 [47] to as many as 1,172 [35] patients. All of the studies (n=79) were published between the years 2020 [6,8,9,12−27,56−69] and 2023 [83−86]. The majority of these studies (n=33) were published in 2020, followed by 2021 (n=31), 2022 (n=15), and 2023 (n=4).
- The 79 articles analyzed presented data from various countries worldwide. Italy accounted for the most articles, with 14 studies [16,19,20,22,23,28,29,36,37,58,66,72,81,86]. This was followed by India with 8 studies [17,33,44,45,50,55,80,83], United States with 7 studies [21,25,,30,31,54,62,79], Brazil with 7 studies [9,47,49,52,56,75,77], and both Egypt and Turkey with 5 studies each [34,38,41,68,71] and [26,39,42,48,64], respectively. Iran [5,53,59,60], Iraq [12,43,84,85], and Spain [27,57,65,67] contributed to 4 studies each. Saudi Arabia was represented by 3 studies [40,46,51], while China [15,35] and Israel each [13,74] had 2 studies. Two studies contain data from multiple European countries [18,70]. The remaining countries—Denmark [14], France [24], Qatar [32], Romania [8], the United Kingdom [6], Colombia [61], Norway [63], Indonesia [69], Afghanistan [73], the Czech Republic [76], Ukraine [78], and Poland [82]—each contributed 1 study, respectively.
- Most of the 79 studies were cross-sectional studies (n=41) [5,12−26,29−31,33,34,37−46,48−51,53−55,84−86] followed by case reports (n=20) [9,61−75,78−81], case series (n=11) [6,8,56−60,76,77,82,83], retrospective studies (n=6) [27,28,35,36,47,52], and case-control studies (n=1) [32].
- The studies contained data from 13,252, of whom 7,509 (56.6%) were females. In most of the studies (n=40), the mean age of the patients was 58.26±11.50 years followed by 41.42±17.32 years (23 studies) and 26.18±18.42 years (12 studies). Three studies did not report the patients’ ages [5,34,35] and 1 study was conducted among newborns [8].
- Only 34 studies have documented patients’ medical history. The majority of these studies documented a history of hypertension (n=26), followed by diabetes (n=20), respiratory diseases and asthma (n=7), cardiovascular disease (n=6), allergies (n=4), and other conditions. Twenty-one studies indicated that patients with COVID-19 were admitted to the hospital. Additionally, 11 studies reported that patients were hospitalized in the ICU, with 5 studies revealing that the patients required ventilation.
- Oral signs and symptoms can be broadly categorized into the following: oral ulcerations, redness and burning sensation, xerostomia, red and white lesions, vesiculobullous lesions, morphological changes of the tongue, taste alteration, gingival and periodontal lesions, and salivary gland disorders. Many patients exhibited multiple signs and symptoms affecting various parts of the oral cavity. Therefore, we have assessed each oral manifestation individually. The most prevalent oral manifestation, observed in 23.8% (n=3,157) of patients, was taste alteration (46 studies). This was followed by oral ulceration in 8.1% (n=1,082 patients in 41 studies), redness and burning sensation in 2.2% (n=297 patients in 33 studies), xerostomia in 12.7% (1,694 patients in 24 studies), red and white lesions in 2.4% (326 patients in 18 studies), vesiculobullous lesions in 0.55% (n=73 patients in 13 studies), morphological changes of the tongue in 2.7% (n=360 patients in 27 studies), gingival and periodontal changes in 3.2% (n=430 patients in 12 studies), and salivary gland disorder in 1.07% (n=143 patients in 3 studies). In the majority of patients (23.6%), the tongue was the most commonly affected area, followed by the oral mucosa (14.7%), lips (4.9%), gingiva and periodontium (3.2%), palate (1.9%), and salivary glands (1.1%).
- Risk of Bias Assessment
- JBI critical appraisal checklists were utilized to assess the risk of bias in cross-sectional studies, case-control studies, case reports, and case series. Of the 79 studies evaluated, 50 (63.7%) demonstrated a low risk of bias, while 23 (29.3%) exhibited moderate bias, and a mere 6 studies (7%) showed a high risk of bias (Table 1) [5,6,8,9,12−86]. The detailed computation of the risk of bias, using the JBI Critical Appraisal Tools, is presented in Tables S1-S4 [5,6,8,9,12−86].
- Descriptive Characteristics of the Oral Lesions
- The prevalence of taste disorders and tongue manifestations was evaluated using data from 46 and 27 studies, respectively. We further subdivided the taste disorders into additional categories. These include complete loss of taste, or ageusia, as reported in 22 studies [12,14,20,23,35,40−42,44,49,53,55,56,58,63,65,67,70,72,74,85], taste alteration or dysfunction, as reported in 19 studies [5,13,16−19,21,22,29,36,38,46,51,54,66,78,84−86], dysgeusia, as reported in 7 studies [24,28,37,50,54,59,60], and amblygeustia, as reported in only one study [15].
- Xerostomia was noted in 22 cross-sectional studies [13,15,22,28,34,37,38,40−42,44−46,48,50−55,84,85] and 2 case reports [60,78].
- Thirteen studies, comprising case reports and case series, reported vesiculobullous lesions [6,9,24,26,29,50,57,59,61,62,70,77,81].
- Ulceration was reported in 41 studies [5,6,24,27−29,31,33,34,37,39−51,53,55,56,61,64,69,73,75−85].
- Eighteen studies reported red and white lesions in COVID-19-positive patients [8,24−26,29,33,36,37,39,43,49,51,53,57,61,68,80,82].
- Twelve studies described the involvement of gingiva and periodontium among patients with COVID-19 [6,26,29,32,38,39,42,44,51,55,59,78].
- In total, 33 studies reported patients who complained of redness and burning sensation [5,6,22,26,28,30,31,33,34,38−44,46,48,50−55,57,59,60,69,78,79,81,82,85].
- Salivary gland disorders [37,38,52] were found in 3 studies.
- The latency time between the emergence of systemic symptoms and oral lesions ranged from 2 weeks prior to 10 days following the onset of systemic symptoms. In the majority of the studies (n=14), systemic symptoms occurred after the appearance of oral symptoms (Table 2) [5,6,8,9,12−86].
- The general treatment protocol, as well as the specific treatment for oral lesions in COVID-19 patients, is outlined in Table S5 [6,8,9,29,43,47,56,57,59−61,64,65,67−71,73,75,77,79,80,82]. Oral lesions typically healed between 7 and 21 days post-emergence. Depending on the severity and cause of the oral lesions, various therapies were prescribed. These included chlorhexidine mouthwash, nystatin, oral fluconazole, topical or systemic corticosteroids, systemic antibiotics, systemic acyclovir, artificial saliva, and photobiomodulation therapy.
- Results of the Meta-Analysis
- Data from 48 studies were meta-analyzed to determine the prevalence of taste alteration, xerostomia, red and white lesions, vesiculobullous lesions, ulceration, burning sensation, and salivary gland involvement.
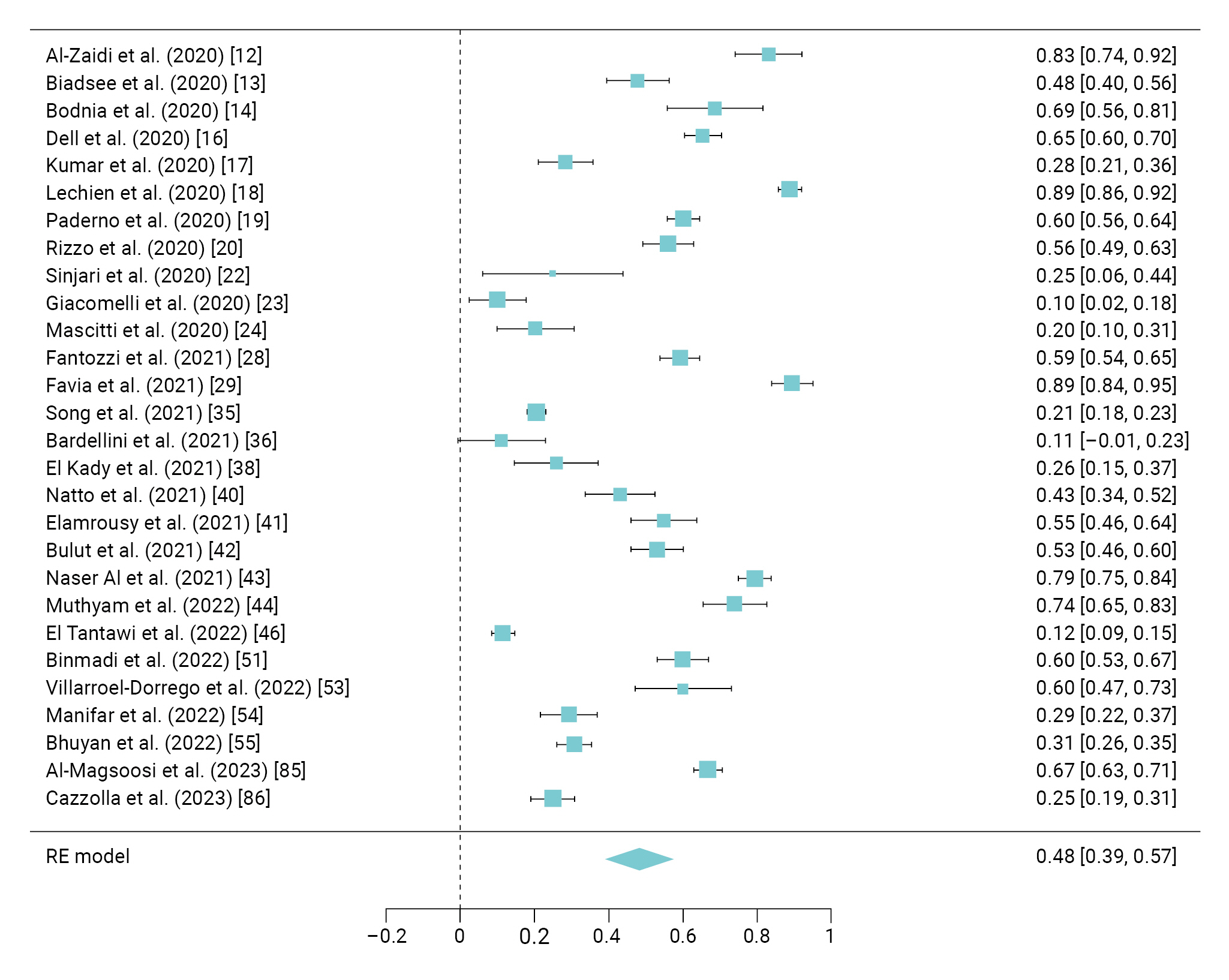
- Among the prevalence studies, 28 separate studies [12−14,16−20,22−24,28,29,35,36,38,40−44,46,51,53−55,85,86] investigated the occurrence of taste alteration in a total of 3,157 COVID-19 patients. The meta-analysis of these studies revealed a combined prevalence rate of 48% (95% confidence interval [CI], 39%–57%). The heterogeneity of these studies was assessed using Cochran Q test and the I2 index, indicating a high degree of heterogeneity with an I2 value of 98.7 (Figure 2).
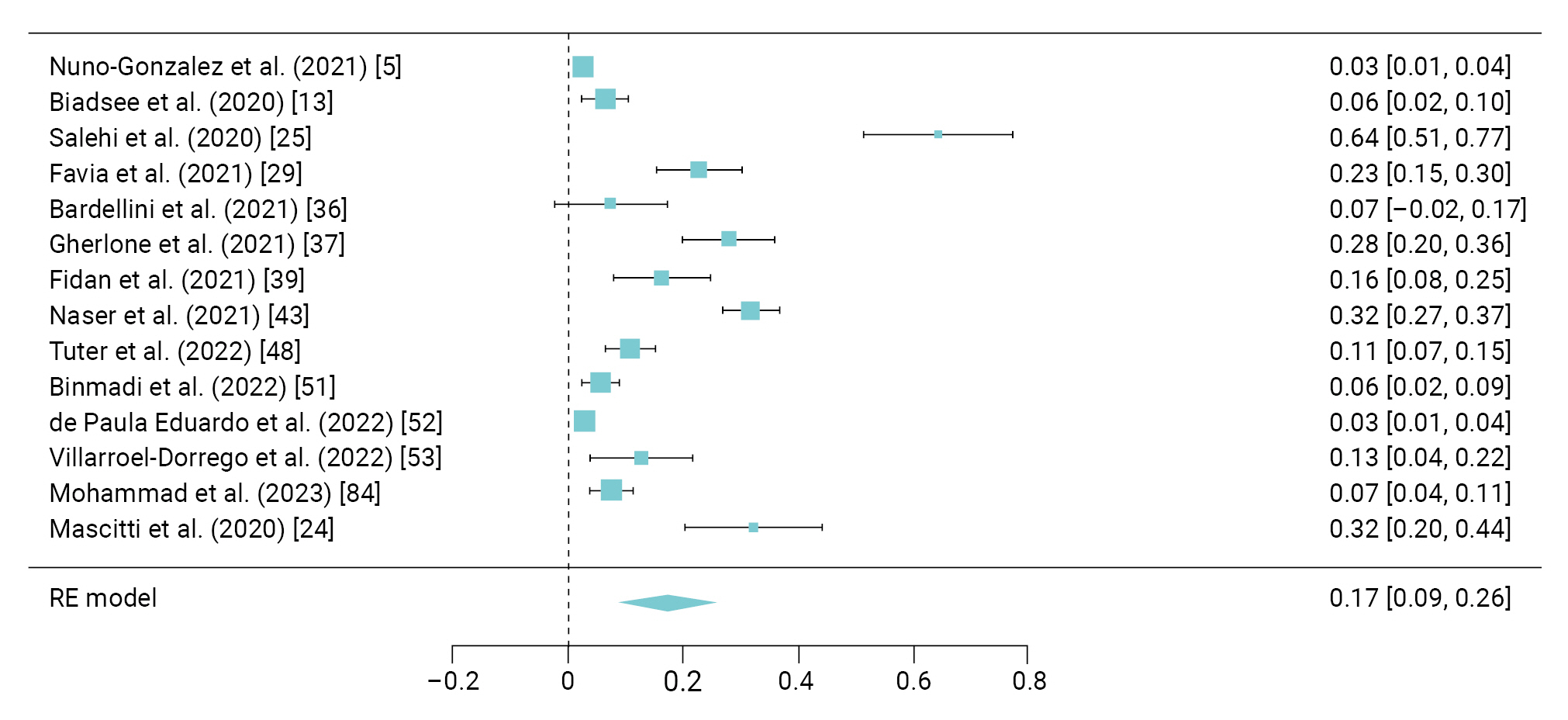
- A total of 327 COVID-19 patients from 14 different studies reported experiencing red and white lesions [5,13,24,25,29,36,37,39,43,48,51−53,84]. These studies revealed a pooled prevalence of these symptoms at 17% (95% CI, 9%–26%), with an I2 value of 98.2% (Figure 3).
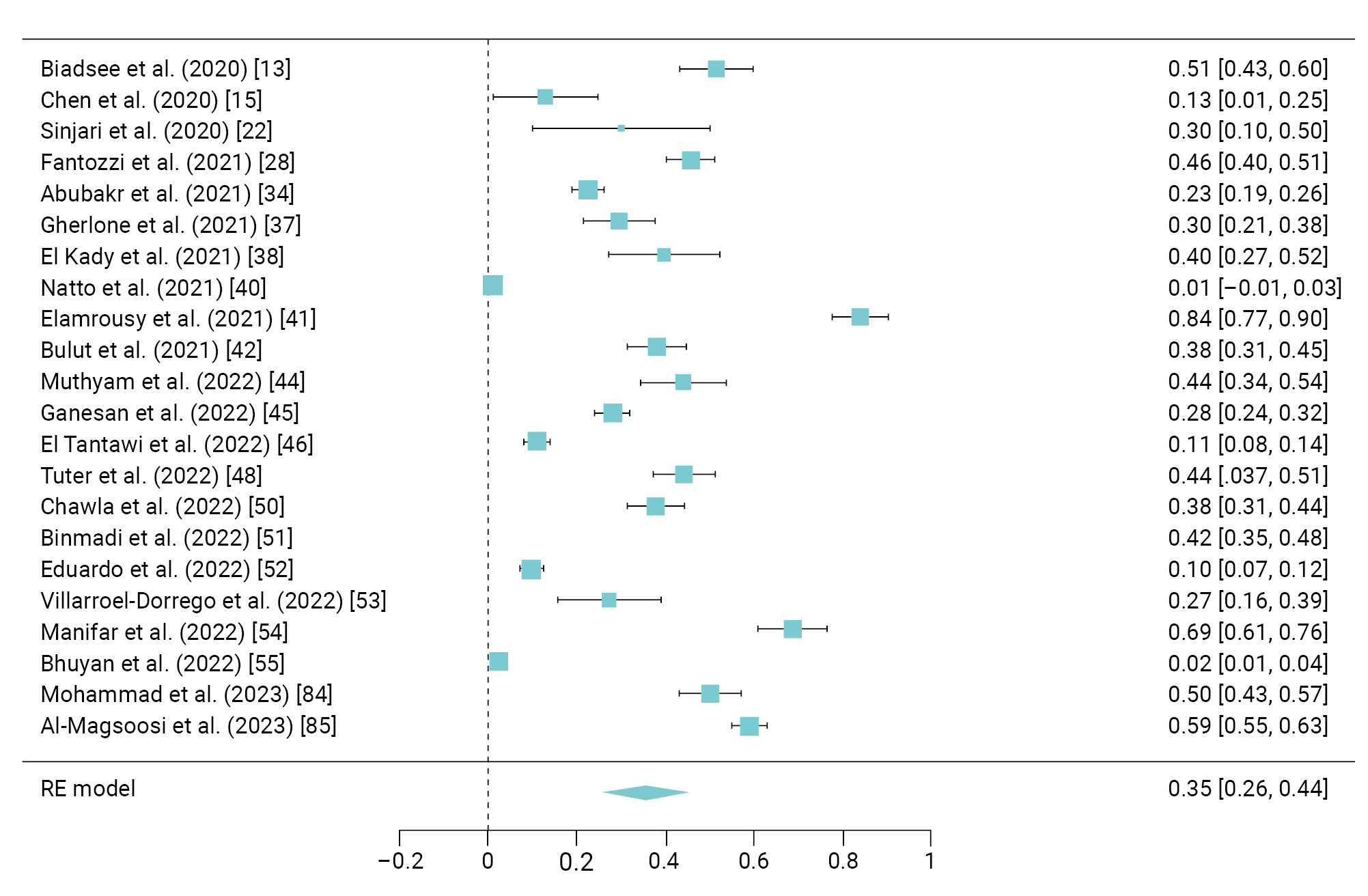
- Xerostomia was reported in 22 studies [13,15,22,28,34,37,38,40−42,44−46,48,50−55,84,85] involving 1,694 COVID-19 patients, showing a pooled prevalence of 35% (95% CI, 26%–44%) and I2=98.7% (Figure 4).
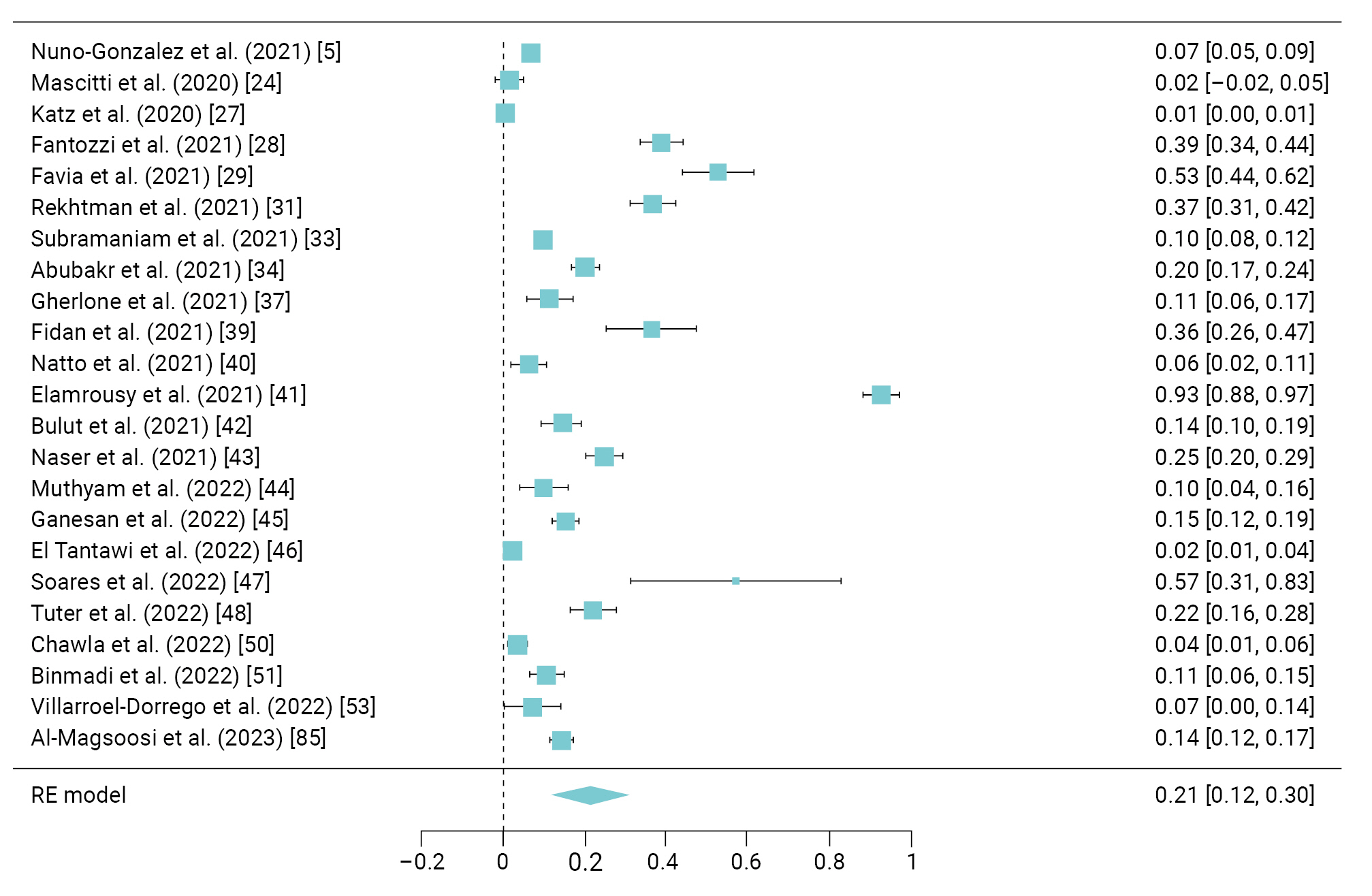
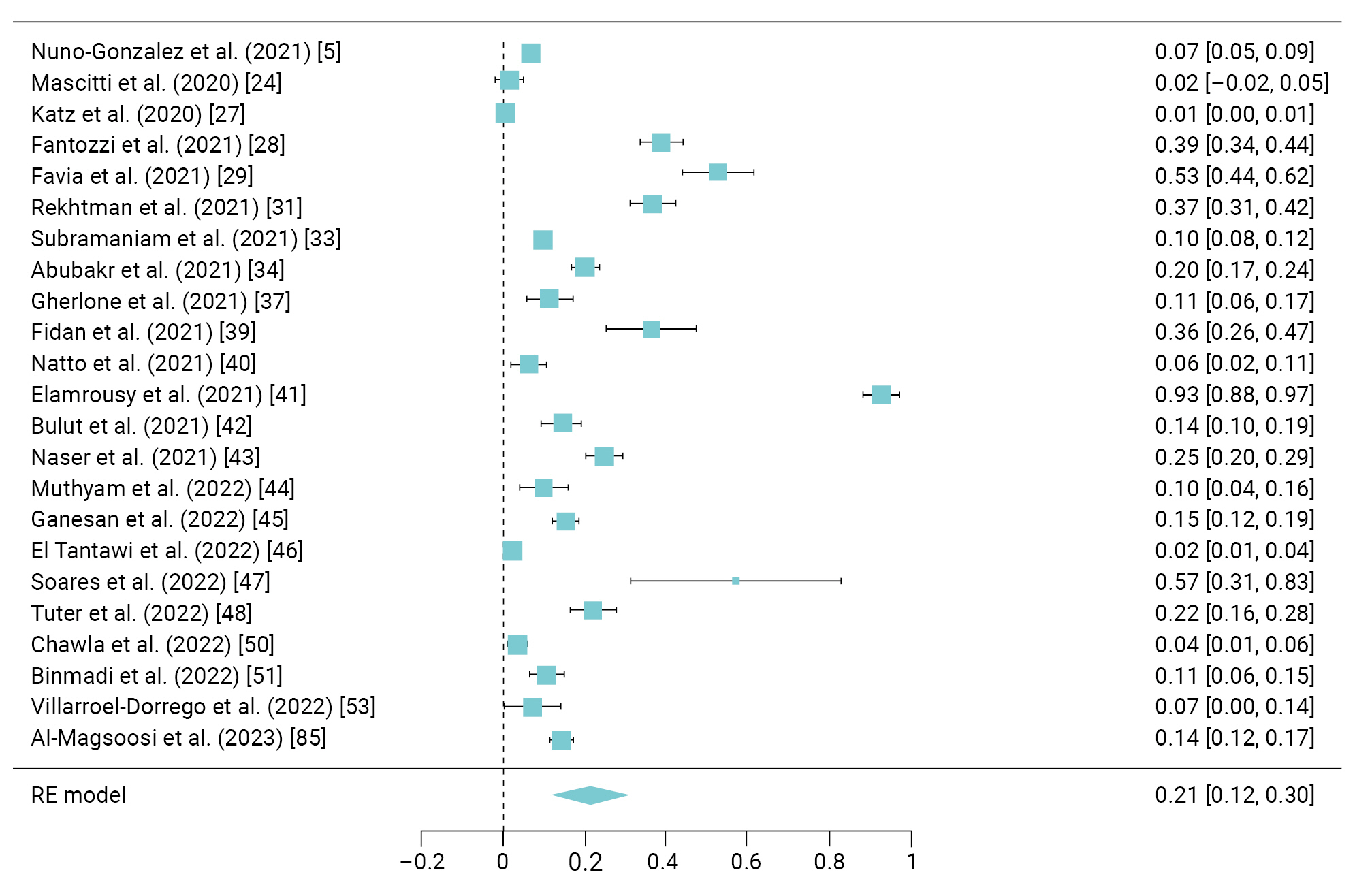
- Ulceration was reported in 23 studies [5,24,27−29,31,33,34,37,39−48,50,51,53,85] involving 1,086 patients, with a a prevalence of 21% (95% CI, 12%–30%) and I2 of 99.62% (Figure 5).
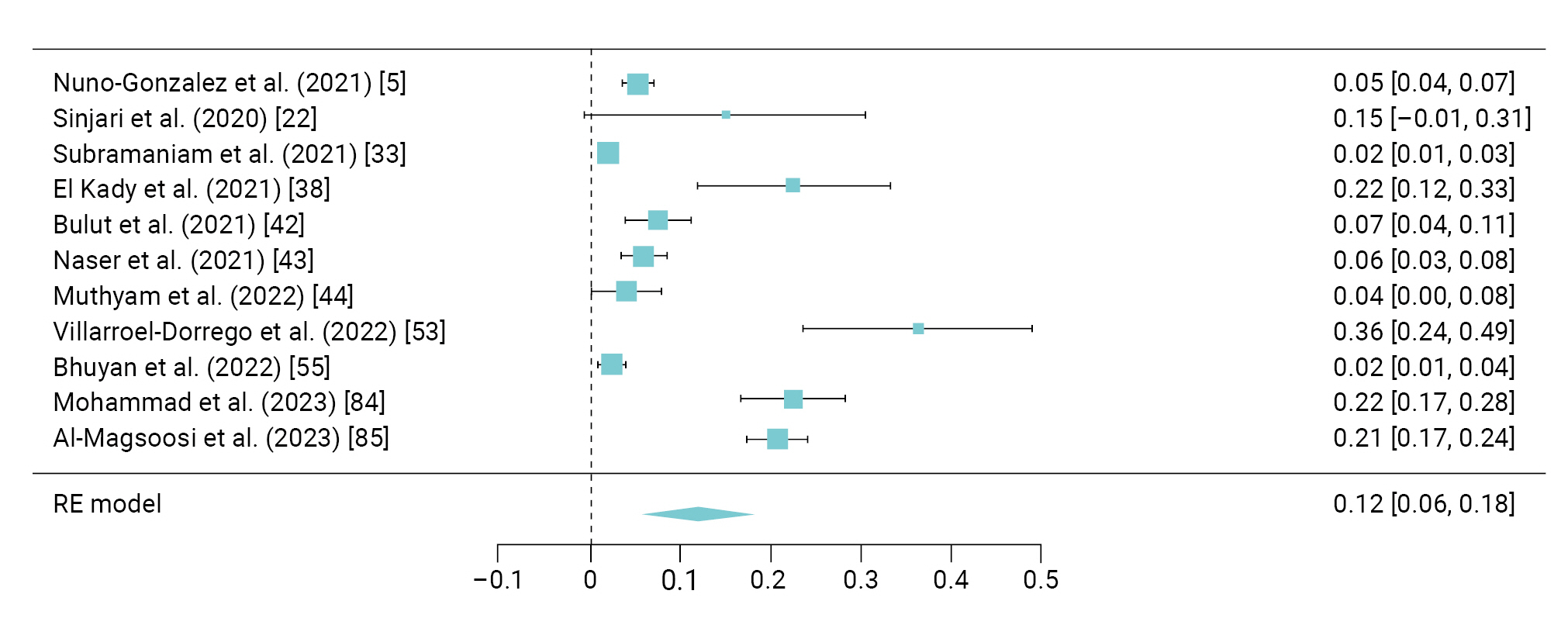
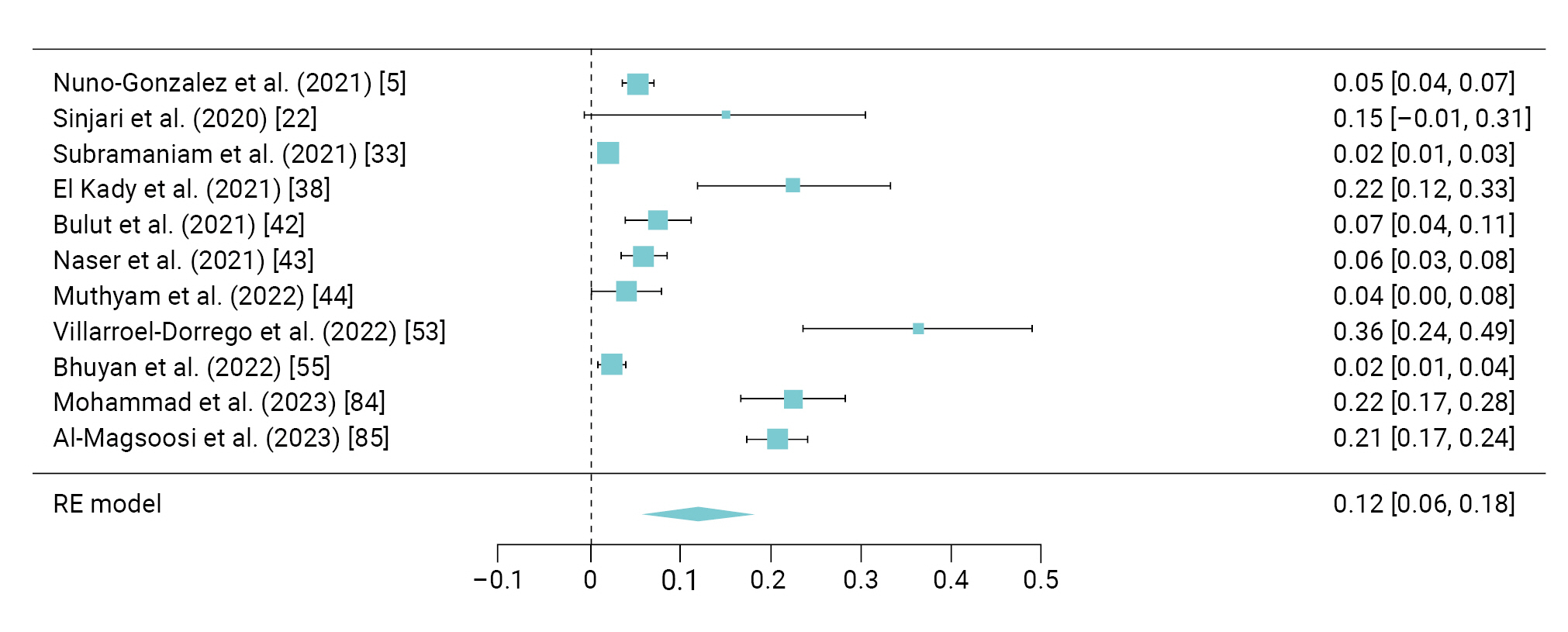
- The pooled prevalence of a burning sensation was reported to be 12% (95% CI, 6%–18%) with I2=98.3%; this symptom was found in 297 patients in 11 studies [5,22,33,38,42−44,53,55,84,85] (Figure 6).
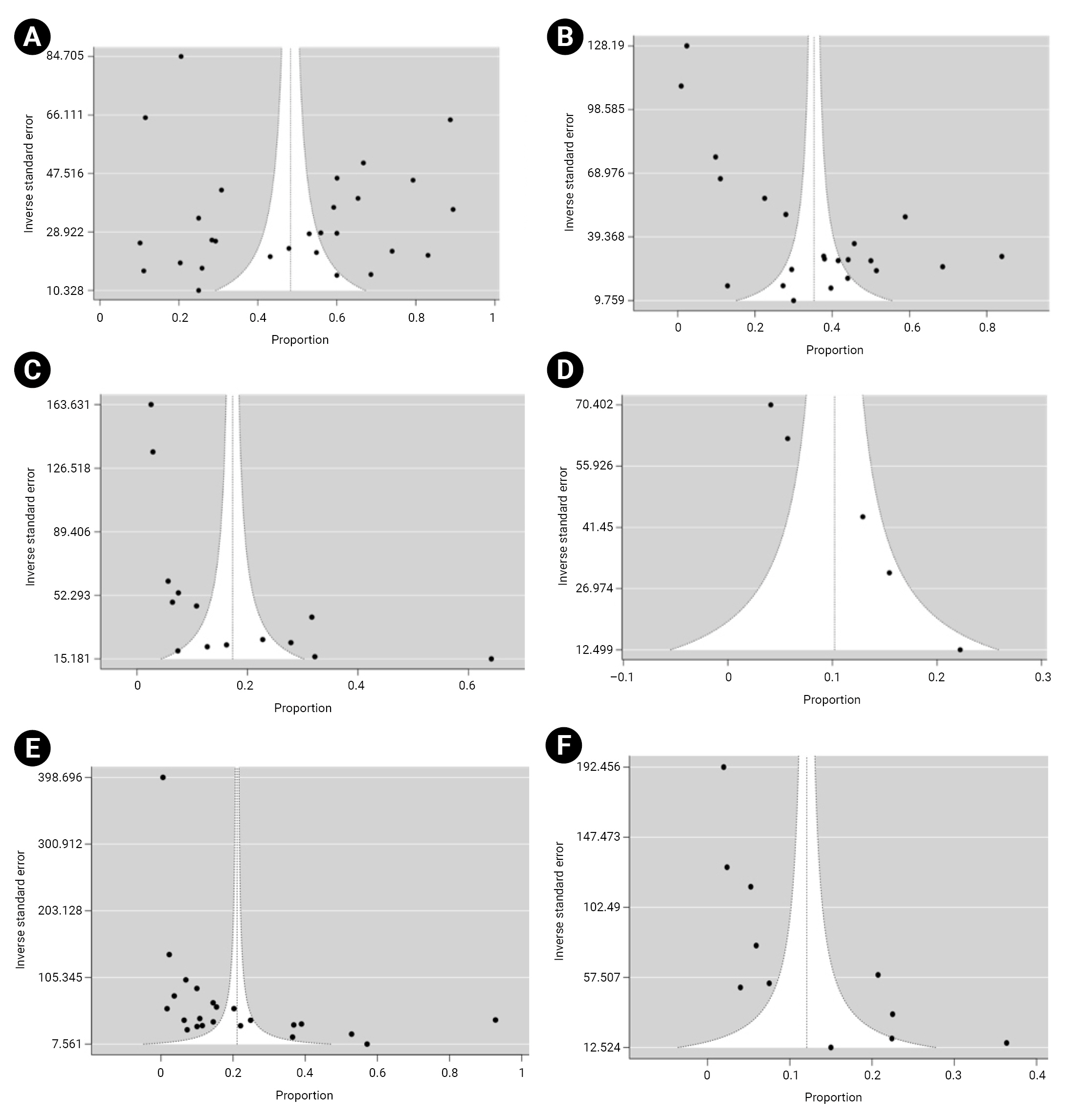
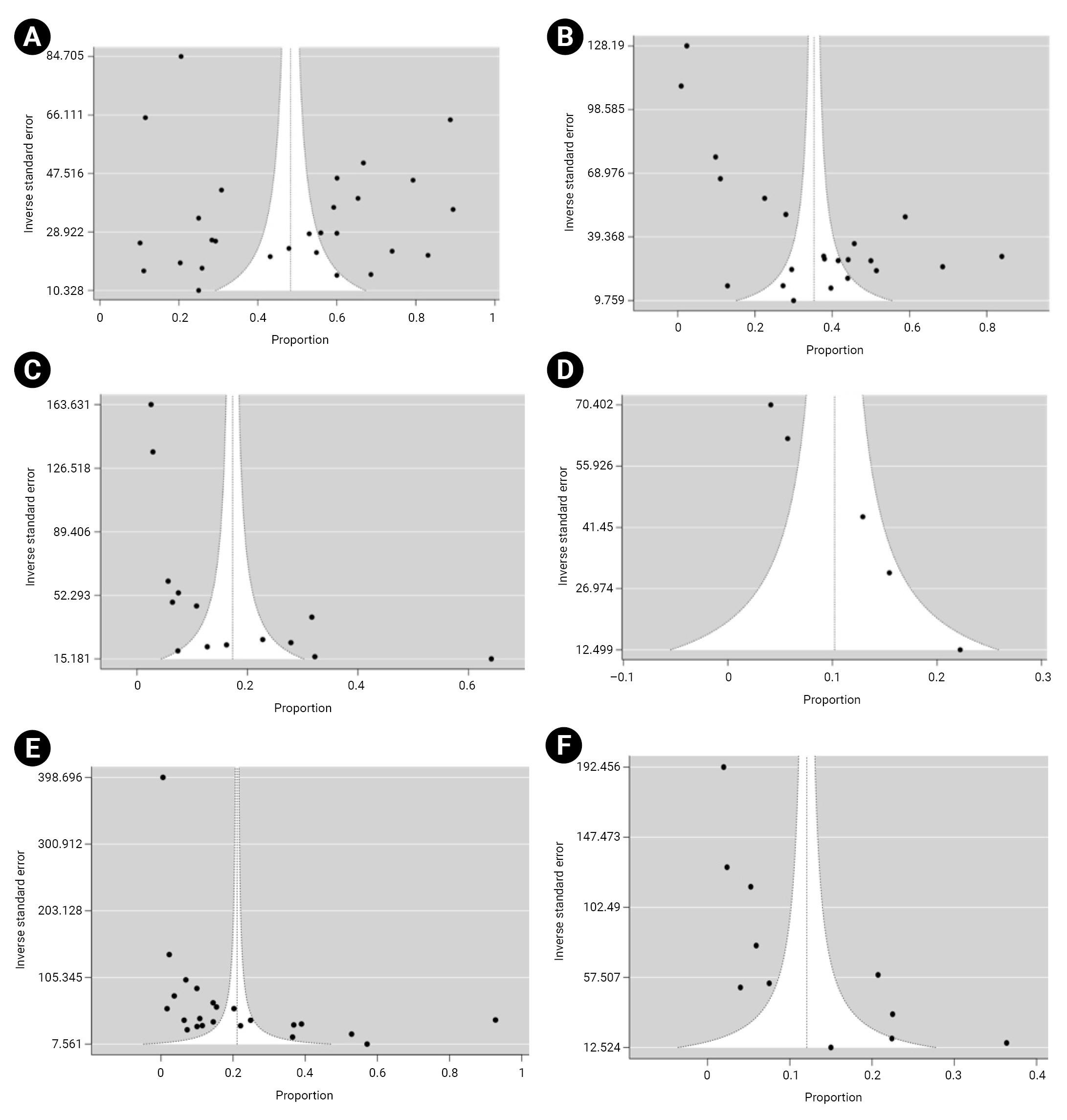
- Vesiculobullous lesions were reported by 73 participants from 5 studies [26,29,36,50,51], with a pooled prevalence of 10% (95% CI, 5%–16%) and an I2 of 98.5% (Figure 7A).
- Salivary gland involvement was reported only in 3 studies [37,38,52] involving 193 patients, showing a pooled prevalence of 32% (95% CI, 22%–41%) and I2=98.7% (Figure 7B).
- Funnel plots demonstrated asymmetry, indicating the presence of high publication bias in the studies (Figure 8).
Results
Taste disorders and tongue manifestation
Xerostomia
Vesiculobullous lesions
Ulceration
Red and white lesions
Periodontal involvement
Redness and burning sensation
Salivary gland involvement
- COVID-19 has emerged as a global public health issue. Initially, it was believed that the absence of oral mucosa involvement distinguished COVID-19 from other viral infections. However, in April 2020, a case report by Chaux-Bodard et al. [87] demonstrated a link between COVID-19 and oral mucosa. The report detailed a 45-year-old woman who experienced painful inflammation of the tongue’s papilla, which eventually healed into an asymptomatic ulcer within 10 days, leaving no scar. This patient also developed a skin lesion on her toe and tested positive for COVID-19 on the eighth day. Since this report, numerous observational studies and case reports have been published, highlighting the involvement of oral mucosa in COVID-19 patients. This systematic review was undertaken to determine the prevalence of oral manifestations in patients with COVID-19.
- SARS-CoV-2 infiltrates human cells in the lower respiratory system using receptors known as ACE2 and transmembrane protease serine 2 [3]. Of these 2, the ACE2 receptor is primarily located in the cells of the lung, liver, kidney, GI tract, and even in the cells of the nasal epithelium and oral mucosa [4]. These cells serve as host cells for the virus, which invades these body cells and triggers an inflammatory response in these organs. This response, in turn, leads to early smell and taste dysfunctions during the disease’s progression [15]. Therefore, the development of oral lesions can occur directly through the effects of the virus replicating in these cells (resulting in SARS-CoV-2-specific lesions) and indirectly as a consequence of potential drug reactions that may occur during the latency period, viral exanthem, due to the physical and psychological stress of COVID-19 or its treatment, or co-infection with other bacterial infections that exacerbate the severity of COVID-19 [59,88]. The involvement of the oral cavity becomes a unique characteristic of COVID-19 [89]. According to Amorim dos Santos et al. [4], the general health deterioration of COVID-19 patients, coupled with extended hospitalization periods and numerous treatment procedures, also increases the likelihood of oral lesions. Chaux-Bodard et al. [87] proposed that oral lesions might emerge as a result of various inflammatory reactions that induce vascular inflammation. Previous reports from Italy and the United Kingdom have noted a temporary association between pediatric inflammatory multisystem syndrome and SARS-COV-2 cases [90]. Certain diseases, such as Kawasaki disease and erythema multiforme, can predispose individuals to oral manifestations. Consequently, we have excluded such conditions from our systematic review.
- Regarding oral lesions, the tongue was the most frequently affected area (n=65), followed by the oral mucosa (n=37), and then the lips (n=19). de Sousa and Paradella [7] identified the palate and tongue, followed by the gums and lips, as the areas most commonly affected in COVID-19 patients. The oral manifestations among COVID-19 patients are described below:
- Taste Disorders
- Numerous studies have indicated that alterations in smell and taste can serve as early signs of COVID-19 infection, playing a crucial role in early diagnosis and decision-making. While these symptoms are not life-threatening, they can significantly impact a patient’s quality of life. Professor C. Hopkins, President of the British Rhinological Society, has noted that the loss of smell or taste may be the sole symptom of COVID-19 [91]. Several public health surveillance organizations, including the European Centre for Disease Prevention and Control, the Centers for Disease Control and Prevention, the WHO [92], and Public Health England, have incorporated the sudden onset of anosmia, ageusia, or dysgeusia into their primary clinical criteria for defining a COVID-19 case [93]. The present systematic review also found that general symptoms typically follow oral symptoms, particularly the loss of taste. The likely reason for taste alteration in COVID-19 patients is the higher expression of the ACE2 receptor in the tongue than in the buccal and gingival tissues. This results in damage to the mucosal epithelial cells of the oral cavity [94,95].
- In the present systematic review, the pooled prevalence of taste alteration was 48%. A recent review by Scotto et al. [89] indicated that the prevalence of taste disorders varied widely across studies, ranging from 1.0% to 93.0%. In a cross-sectional study by Al-Zaidi and Badr [12], 83.08% of COVID-19 patients experienced taste dysfunction. For 50% of these patients, taste returned within a week, while for 25% it took less than a week, for 18.75% it took within 2 weeks, and for 6.25% it took within 3 weeks. In their living systematic review (LSR), Amorim dos Santos et al. [4] identified taste disorders as the most common oral symptom in this population, with a prevalence of 45%. However, in their subsequent LSR, the prevalence dropped to 38%. They noted that the prevalence of taste disorders among COVID-19 patients varies geographically, from 14% in Africa to 49% in Europe [96]. Yan et al. [21] reported taste loss in 71% of COVID-19-positive subjects, and found a strong association between taste loss and COVID-19 positivity (odds ratio, 10.2; 95% CI, 4.74–22.1). In a study by Biadsee et al. [13], 52% of participants reported changes in taste sensation, with 52 patients noting a change in spicy taste perception, 54 in salty taste, 53 in sour taste, and 61 in sweet taste. Bodnia and Katzenstein [14] found that 70% of patients experienced a total loss of taste, which resolved within 1 to 3 weeks for 78% of patients and within 3 to 6 weeks for 22%. A meta-analysis by Tong et al. [97] revealed that 43.93% of individuals noted changes in taste. Another meta-analysis by Nijakowski et al. [98] estimated the prevalence of taste alterations to be around 54.73% (95% CI, 46.28%–63.04%).
- Three studies in our systematic review, conducted by Favia et al. [29], Bardellini et al. [36], and Binmadi et al. [51], reported geographic tongue. Bardellini et al. [36] carried out a retrospective cohort study on pediatric patients and identified the most common oral lesions as oral pseudomembranous candidiasis (n=2), coated tongue (n=2), taste alteration (n=3), and geographic tongue (n=1). Notably, the occurrence of geographic tongue coincided with a high fever, as reported by the patient's mother. The etiopathogenesis of geographic tongue remains unclear, but some researchers have suggested a link with several non-genetic multifactorial factors, including viral infections [99].
- Vesiculobullous Lesions and Ulceration
- Thirteen studies, encompassing both case reports and case series [6,9,24,26,29,50,57,59,61,62,70,77,81], documented vesiculobullous lesions. Among these, 5 cross-sectional studies revealed a combined prevalence of 10%.
- Ulceration was reported by 23 studies [5,24,27−29,31,33,34,37,39−48,50,51,53,85], involving 1,086 patients with a pooled prevalence of 21%. This is slightly higher than the value reported in the meta-analysis by Aragoneses et al. [100] (10%). Favia et al. [29] provided a detailed description of the histological aspects of oral SARS-CoV-2-related lesions, identifying ulcers as the most common oral manifestation, in 52.8% of patients. Martin Carreras-Presas et al. [59] reported 3 cases of ulcers, 1 of which was confirmed to be infected with SARS-CoV-2, while the other 2 were suspected cases. These lesions were similar in appearance to herpes simplex infection, but this was not confirmed by biopsy. Halboub et al. [101] conducted a review and found that painful oral ulcers were the most common orofacial manifestation in patients with COVID-19. Bullous lesions on the palate and oral mucosa were found by Cruz Tapia et al. [57] and Dalipi et al. [70], respectively. Orilisi et al. [102] conducted a systematic review and reported that oral ulcers, cheilitis, and tongue lesions were more common in patients prior to hospitalization. In contrast, perioral pressure ulcers, macroglossia, blisters, and oral candidiasis were more frequently observed in patients during their hospital stay.
- The mechanism behind ulcer formation is thought to involve an elevated level of tumor necrosis factor-α in COVID-19 patients, which can trigger the chemotaxis of neutrophils to the oral mucosa, leading to the development of aphthous-like lesions. Other potential causes for these lesions in COVID-19 patients could be stress and immunosuppression, both secondary effects of the infection [9].
- Eighteen studies have reported the presence of red and white lesions in patients who tested positive for COVID-19, with a pooled prevalence of 17% [8,24−26,29,33,36,37,39,43,49,51,53,57,61,68,80,82]. The variety of red and white lesions documented in these studies include cheilitis and oral lichenoid reactions [24], white plaques on the inner oral mucous layer [25,37,43,68,80], rashes and erythema [26], candidiasis [9,29,36,49,51,53], reddish-white spots on the palate [33,57], erythema and lichen planus [39], angular cheilitis [53,82], and reddish plaques on the lower lip [61].
- Xerostomia was observed in 22 cross-sectional studies [13,15,22,28,34,37,38,40−42,44−46,48,50−55,84,85] and 2 case reports [60,78]. These studies reported a combined prevalence of 35%, a figure slightly lower than the 44% prevalence (95% CI, 36%–52%) found in a recent meta-analysis by Aragoneses et al. [100]. In research conducted by Biadsee et al. [13], 56% of patients reported experiencing xerostomia, as determined by the question, “Do you feel the need to drink more (dry mouth)?”. In the revised version of the LSR by Amorim Dos Santos et al. [96], xerostomia was the most frequently reported oral symptom in COVID-19 patients, whereas tassate disturbances were the primary feature in the original LSR [4]. In a meta-analysis by Nijakowski et al. [98], xerostomia was prevalent in 37.58% (95% CI, 26.35%–49.53%) of COVID-19 patients.
- Twelve studies (15%) [6,26,29,32,38,39,42,44,51,55,59,78] reported the prevalence of gingivitis and periodontitis. The gingival symptoms identified in COVID-19 patients from our systematic review included gingivitis [29], desquamative gingivitis [6,59], ulceronecrotic gingivitis [29], and gingival bleeding [6,38,42,44,55,78]. Two studies reported instances of periodontitis [32] and necrotizing periodontal disease [51].
- Red and/or swollen lips was observed by Halepas et al. [30] in 48.9% of patients. Other findings related to lip involvement in COVID-19 patients included pale lips [33], reddish plaques on the lower lip [61], nodules on the lower lip [10], and reddish macules [42]. In terms of palatal findings among COVID-19 patients, there were reddish-white spots on the palate [33], palate ulcerations [6,39,56,59,60,81,82], a white coating on the palate [43,68], bullae on both the left and right palatal mucosa [57], an erythematous surface on the hard palate [64], and an angioma-type lesion on the right side of the palate [82].
- Although we have attempted to summarize the findings of studies that report oral manifestations in COVID-19 patients, a significant limitation of this systematic review is the absence of a temporal dimension. We cannot definitively state that these oral manifestations are directly linked to COVID-19, or if they are indirect manifestations of other factors such as stress, immunosuppression, and/or medications. Another limitation is the absence of a definitive diagnosis, as most of the cases included in the review did not undergo a biopsy to confirm the diagnosis.
Discussion
Red and white lesions
Xerostomia
Gingival and periodontal involvement
Other findings
Limitations
- Our systematic review showed a relatively high prevalence of oral manifestations, specifically taste alteration (48%), followed by dry mouth (35%), ulceration (21%), and red and white lesions (17%). These patients exhibit a variety of oral symptoms that could aid clinicians in the early detection of the disease. It is crucial to recognize the signs and symptoms of COVID-19 for a prompt diagnosis and an improved prognosis. Dental practitioners can play a significant role not only in preventing the transmission of COVID-19 but also in interrupting the disease's progression. Increasing awareness of these symptoms is vital for the early diagnosis and treatment of this deadly disease.
Conclusion
- • The present systematic review shows a higher prevalence of oral manifestations among COVID-19 patients, specifically taste alterations, followed by xerostomia, ulceration, and red and white lesions.
- • COVID-19 patients show various oral manifestations that may help clinicians detect the disease early in its course.
- • Identifying the oral signs and symptoms of COVID-19 is crucial for initiating early diagnosis and treatment of this deadly disease; therefore, increasing awareness of these symptoms is important.
HIGHLIGHTS
Supplementary Material
Table S1.
Table S2.
Table S3.
Table S4.
Table S5.
-
Ethics Approval
Not applicable.
-
Conflicts of Interest
The authors have no conflicts of interest to declare.
-
Funding
None.
-
Availability of Data
The datasets are not publicly available but are available from the corresponding author upon reasonable request.
-
Authors’ Contributions
Conceptualization: AG, KS, AP; Data curation: AG, AA; Formal analysis: AG, AP; Investigation: AG, KS, AA; Methodology: AG, KS, AA, AP, RC; Project administration: AG, KS; Software: AG, AP; Supervision: AG, AA; Validation: AG, AP; Visualization: AG, RC; Writing–original draft: AG; Writing–review & editing: authors. All authors read and approved the final manuscript.
Article information


| No. | Study | Year of publication | Study location | Study design | Sample size (n) | Sex | Mean age (range, y) | Study duration | Medical history | Admission in the ICU | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Nuno-Gonzalez et al. [5] | 2021 | Spain | Cross-sectional | 666 | - | 55.7 (40–70) | April 10–25, 2020 | - | History of hospitalization | Low |
| 2 | Al-Zaidi and Badr [12] | 2020 | Iraq | Cross-sectional | 65 | M, 41.6%; F, 58.4% | 41.2 (11–80) | April 5, 2020–May 17, 2020 | - | - | Moderate |
| 3 | Biadsee et al. [13] | 2020 | Israel | Cross-sectional | 140 | M, 58; F, 70 | 36.5 (18–73) | March 25, 2020–April 15, 2020 | - | - | Moderate |
| 4 | Bodnia and Katzenstein [14] | 2020 | Copenhagen, Denmark | Cross-sectional | 51 | F, 22; M, 28 | 45 (16–62) | March 2020 | - | - | Moderate |
| 5 | Chen et al. [15] | 2020 | China | Cross-sectional | 31 | M, 15; F, 16 | 60.6 (18–86) | February 28, 2020–March 4, 2020 | - | - | Moderate |
| 6 | Dell’Era et al. [16] | 2020 | Italy | Cross-sectional | 355 | M, 54% | 45 (51–60) | March 10–30, 2020 | Cardiovascular disease, allergic (sinusitis) | - | Low |
| 7 | Kumar et al. [17] | 2021 | India | Cross-sectional | 141 | M, 58.9%; F, 41.1% | 15.2 (10–19) | May–August 2020 | - | - | Low |
| 8 | Lechien et al. [18] | 2020 | Europe (multi center) | Cross-sectional | 417 | F, 263; M, 154 | 36.9 (19–77) | - | Allergic rhinitis, asthma, hypertension, hypothyroidism | Hospitalization of severe cases | Low |
| 9 | Paderno et al. [19] | 2020 | Italy | Cross-sectional | 508 | M, 56%; F, 44% (55±15 y)a) | 55 (40–70) | March 27–April 1, 2020 | - | Hospitalization of severe cases | Low |
| 10 | Boscolo-Rizzo et al. [20] | 2020 | Italy | Cross-sectional | 202 | F, 55.1%; M, 44.9% | 56 (20–89) | March 19–22, 2020 | - | - | Low |
| 11 | Yan et al. [21] | 2020 | California, USA | Cross-sectional | 59 and 203 (COVID-19 +ve and –ve) | M & F, 49.2% (COVID-19 +ve): M, 34%; F, 65% (COVID-19 –ve) | 54 (18–80) | March 3–29, 2020 | Allergic rhinitis, immunocompromised state, hypertension, DM, cardiac disorders, cancer, CLD, history of head trauma, neurological disease | Hospitalization of severe cases | Low |
| 12 | Sinjari et al. [22] | 2020 | Italy | Cross-sectional | 20 | - | 69.2 (39–81) | May 2020–June 2020 | DM, cardiovascular conditions | - | Low |
| 13 | Giacomelli et al. [23] | 2020 | Italy | Cross-sectional | 59 | M, 40%; F, 60% | 60 (40–74) | March 19, 2020 | - | - | Moderate |
| 14 | Mascitti et al. [24] | 2020 | France | Cross-sectional | 59 | M:F, 3:1 | 57.6 (49–69) | March 31, 2020 | - | - | Moderate |
| 15 | Salehi et al. [25] | 2020 | Iran | Cross-sectional | 53 | M, 43.4%; F, 56.6% | 63.1 (27–90) | March 1, 2020–April 30, 2020 | Cardiovascular diseases (52.83%), DM (37.7%), chronic kidney disease (20.7%) | - | Low |
| 16 | Askin et al. [26] | 2020 | Turkey | Cross-sectional | 210 | M, 58.6%; F, 41.4% | 57.4 (20–75) | April 2020 | Comorbidities | 29 in ICU, 129 in wards | Moderate |
| 17 | Katz and Yue [27] | 2021 | USA | Retrospective study | 889 | F, 509; M, 386 | 18–34 | Registry study | - | - | Moderate |
| 18 | Fantozzi et al. [28] | 2020 | Italy | Retrospective study | 326 | M, 52.3%; F, 47.7% | 57 (48–67) | March 6, 2020–April 30, 2020 | Hypertension (n=29), chronic pulmonary disease (n=11), DM (n=10), cardiovascular disease (n=9), cancer (n=5) | Hospitalized (median, 12.5 d) | High |
| 19 | Favia et al. [29] | 2021 | Bari, Italy | Cross-sectional | 123 | M:F, 1.3:1 | Median, 72 | October 2020–December 2020 | - | History of hospitalization and ICU | Moderate |
| 20 | Halepas et al. [30] | 2021 | New York, USA | Cross-sectional | 47 | M, 51.1%; F, 48.9% | 9.0 (1.3–20) | March 15–June 1, 2020 | - | History of hospitalization, ICU | Moderate |
| 21 | Rekhtman et al. [31] | 2021 | New York, USA | Cross-sectional | 296 | M, 71%; F, 29% | 64 (50–77) | May 11, 2020–June 15, 2020 | CAD, 23%; congestive heart failure, 14%; asthma, 9%; COPD, 14%; DM, 34%; hypertension, 71% | History of hospitalization | Low |
| 22 | Marouf et al. [32] | 2021 | Qatar | Case control | Case, 40; control, 528 | Case: M, 50%; F, 50% | Case, 53.6; control, 41.5 | February–July 2020 | DM: case, 42.5%; control, 27.8% | Hospitalization and ICU admission | Low |
| Control: M, 54.9%; F, 45.1% | |||||||||||
| 23 | Subramaniam et al. [33] | 2021 | India | Cross-sectional | 713 | M:F, 6:3 | 69 (60–81) | May 2020–June 2020 | DM, hypertension | - | Moderate |
| 24 | Abubakr et al. [34] | 2021 | Egypt | Cross-sectional | 573 | F, 408; M, 165 | 36.19 (30–45) | May 1, 2020–July 1, 2020 | - | - | Low |
| 25 | Song et al. [35] | 2021 | China | Retrospective | 1172 | - | - | December 2019 | - | History of hospitalization | Low |
| 26 | Bardellini et al. [36] | 2021 | Italy | Retrospective | 27 | M:F, 19:8 | 4.2 y (3 mo–14 y) | March–April 2020 | - | - | High |
| 27 | Gherlone et al. [37] | 2021 | Italy | Cross-sectional | 122 | M, 75.4%; F, 24.6% | 62.5 (53.9–74.1) | July 23, 2020–September 7, 2020 | CAD, DM, chronic kidney disease, active neoplasia, COPD | History of hospitalization and ICU and ventilation | High |
| 28 | El Kady et al. [38] | 2021 | Egypt | Cross-sectional | 58 | M, 53.4%; F, 46.6% | 18–46 | May 15, 2020–June 10, 2020 | - | History of hospitalization | High |
| 29 | Fidan et al. [39] | 2021 | Turkey | Cross-sectional | 74 | M, 66.2%; F, 33.8% | 51.6 (28–68) | April–October 2020 | - | Hospitalized | High |
| 30 | Natto et al. [40] | 2021 | Saudi Arabia | Cross-sectional | 109 | M, 67%; F, 33% | 39.3 (18–56) | July–October 2020 | DM (10.1%), hypertension (7.3%), asthma and arthritis (1.7%) | - | Moderate |
| 31 | Elamrousy et al. [41] | 2021 | Egypt | Cross-sectional | 124 | M, 74.2%; F, 25.8% | 50.32±12.47a) | September 2, 2020–June 10, 2021 | DM (n=52), hypertension (n=16), cardiac disease (n=8), renal disease (n=4), liver disease (n=4) | Hospitalized | Moderate |
| 32 | Bulut et al. [42] | 2021 | Turkey | Cross-sectional | 200 | M, 75; F, 125 | 38 (20–70) | September 2020–March 2021 | - | Hospitalized (11.5%) | Low |
| 33 | Naser et al. [43] | 2021 | Iraq | Cross-sectional | 338 | M, 59%; F, 41% | 45 | August 2020–March 2021 | Respiratory diseases, DM, hypertension, heart disease, urogenital diseases | Hospitalized | Moderate |
| 34 | Muthyam et al. [44] | 2022 | India | Cross-sectional | 100 | M, 51%; F, 49% | More than 35 y, 54%; less than 35 y, 46% | - | Immunocompromised state, multidrug therapy | Hospitalization | Low |
| 35 | Ganesan et al. [45] | 2022 | India | Cross-sectional | 500 | M, 73.4%; F, 26.6% | 53.46±17.50a) | - | - | - | Low |
| 36 | El Tantawi et al. [46] | 2022 | Multicountry study (Saudi Arabia) | Cross-sectional | 434 | M, 41.5%; F, 58.5% | 18–23 | August 2020–January 2021 | Cancer, COPD | - | Moderate |
| 37 | Soares et al. [47] | 2022 | Brazil | Retrospective | 14 | M, 71.5%; F, 38.5% | 58 (20–65) | - | - | - | Low |
| 38 | Tuter et al. [48] | 2022 | Turkey | Cross-sectional | 204 | M, 37.3%; F, 62.7% | 53.3 (18–70) | February–March 2021 | DM, hypertension, immunosuppression | Hospitalization, ICU | Low |
| 39 | Schwab et al. [49] | 2022 | Brazil | Cross-sectional | 154 | M, 59.7%; F, 40.3% | 54.60 (20–88) | January 13, 2021–May 28, 2021 | - | Hospitalization, ICU, ventilation | Moderate |
| 40 | Chawla et al. [50] | 2022 | India | Cross-sectional | 217 | M, 70%; F, 30% | 56 (18–60) | September–December 2020 | DM, hypertension, CAD, bronchial asthma | - | High |
| 41 | Binmadi et al. [51] | 2022 | Saudi Arabia | Cross-sectional | 195 | M, 25%; F, 75% | 26 (18–34) | March 2020–March 2022 | Immunosuppression, hormonal modulation | Hospitalization, ICU, ventilation | Moderate |
| 42 | Eduardo et al. [52] | 2022 | Brazil | Retrospective | 519 | M, 68.2%; F, 31.8% | 51–80 | May 2020–February 2021 | - | ICU | Moderate |
| 43 | Villarroel-Dorrego et al. [53] | 2022 | Spain | Cross-sectional | 55 | M, 54.5%; F, 45.5% | 51 (1–89) | - | - | - | Moderate |
| 44 | Manifar et al. [54] | 2022 | Iran | Cross-sectional | 140 | M, 44.2%; F, 55.8% | 53.78 (15–92) | September 1, 2020–October 17, 2020 | - | Hospitalization | Moderate |
| 45 | Bhuyan et al. [55] | 2022 | India | Cross-sectional | 169 (1st wave), 211 (2nd wave) | 1st wave: M, 35.5%; F, 64.5% | 63±17 and 57±18 (1st and 2nd wave)a) | - | Comorbidities | Hospitalization, ventilator | Moderate |
| 2nd wave: M, 45.5%; F, 55.5% | |||||||||||
| 46 | Mohammad et al. [84] | 2023 | Iraq | Cross-sectional | 200 | M, 81; F, 119 | 36.69 (16–78) | September–December 2021 | - | - | Moderate |
| 47 | Al-Magsoosi et al. [85] | 2023 | Iraq | Cross-sectional | 574 | M, 196; F, 378 | 18–78 | October 2021–April 2022 | - | - | Low |
| 48 | Cazzolla et al. [86] | 2023 | Italy | Cross-sectional | 1,155 | M, 57%; F, 43% | M, 59±13; F, 56± 16a) | March 15, 2020–April 15, 2021 | - | - | Moderate |
| 49 | Sinadinos and Shelswell [6] | 2020 | United Kingdom | Case series | 3 | M:F, 2:1 | 56 (45–61) | DM, hypertension (case 2); obesity (case 3) | - | Low | |
| 50 | Dima et al. [8] | 2020 | Romania | Case series | 3 | M:F, 2:1 | Newborns | May 2020 | Diaper erythema | Neonatology ward | Low |
| 51 | Brandao et al. [56] | 2021 | Brazil | Case series | 8 | M, 5; F, 3 | 53 (28–83) | - | Hypertension, COPD (case 1); DM, obesity, renal failure, bariatric surgery, fibromyalgia (case 2); obesity, Parkinson disease, hypertension, COPD (case 3); DM, hypertension (case 4) | Hospitalization | Low |
| 52 | Cruz Tapia et al. [57] | 2020 | Latin America | Case series | 4 | F:M, 3:1 | 47.2 (41–54) | - | - | Case 2, hospitalized | Low |
| 53 | Vaira et al. [58] | 2020 | Italy | Case series | 72 | M, 27; F, 45 | 49.2 (18–67) | March 31, 2020–April 6, 2020 | History of head trauma, allergic rhinitis, chronic rhino sinusitis, psychiatric or neurological disorders | - | Low |
| 54 | Martin Carreras-Presas et al. [59] | 2021 | Spain | Case series | 3 | M:F, 2:1 | 55 (56–65) | Last week of March–First week of April 2020 | DM, hypertension (case 2); obesity, hypertension (case 3) | Case 3, hospitalization | Low |
| 55 | Rodriguez et al. [60] | 2022 | Spain | Case series | 3 | F:M, 2:1 | 68 (53–78) | - | - | Case 1, home quarantine; cases 2 & 3, hospitalization | Low |
| 56 | Corchuelo and Ulloa [61] | 2020 | Colombia | Case report | 1 | F | 40 | - | - | - | Low |
| 57 | Amorim Dos Santos et al. [9] | 2020 | Brazil | Case report | 1 | M | 67 | March 31, 2020 | CAD, autosomal dominant polycystic kidney disease, and kidney transplant, immunosuppression, venous thromboembolism | Hospitalization in ICU | Low |
| 58 | Eghbali Zarch and Hosseinzadeh [62] | 2021 | Iran | Case report | 1 | F | 56 | October 2020 | - | - | Low |
| 59 | Hjelmesaeth and Skaare [63] | 2020 | Norway | Case report | 1 | F | 60 | - | - | - | Low |
| 60 | Cebeci Kahraman and Caskurlu [64] | 2020 | Turkey | Case report | 1 | M | 51 | March 18, 2020 | - | - | Low |
| 61 | Smith et al. [65] | 2020 | USA | Case report | 1 | M | 21 | March 19, 2020 | - | - | Low |
| 62 | Maniaci et al. [66] | 2020 | Italy | Case report | 1 | M | 15 | - | - | - | Low |
| 63 | Melley et al. [67] | 2020 | Pennsylvania, USA | Case report | 1 | F | 59 | May 2020 | - | - | Low |
| 64 | Riad et al. [68] | 2022 | Egypt | Case report | 1 | F | 47 | - | Cardiovascular disease, DM | - | Low |
| 65 | Putra et al. [69] | 2020 | Indonesia | Case report | 1 | M | 29 | - | Cardiovascular diseases | - | Low |
| 66 | Dalipi et al. [70] | 2021 | Europe | Case report | 1 | M | 17 | - | - | - | Low |
| 67 | Eita [71] | 2021 | Egypt | Case report | 1 | F | 31 | - | Irritable bowel syndrome, atopy | - | Low |
| 68 | Cirillo and Colella [72] | 2021 | Italy | Case report | 1 | F | 36 | March 2020 | - | - | Low |
| 69 | Nejabi et al. [73] | 2021 | Afghanistan | Case report | 1 | M | 62 | - | - | - | Low |
| 70 | Klein et al. [74] | 2021 | Israel | Case report | 1 | F (pregnant) | 40 | - | - | - | Low |
| 71 | Ramires et al. [75] | 2021 | Brazil | Case report | 1 | F | 50 | - | Obesity, hypertension, type 2 DM | Hospitalization, ventilation | Low |
| 72 | Hockova et al. [76] | 2021 | Czech Republic | Case series | 3 | M:F, 3:0 | 62 | Arterial hypertension, hypercholesterolemia, GERD (case 1); arterial hypertension, history of MI, septic shock (case 2) | ICU | Low | |
| 73 | Teixeira et al. [77] | 2021 | Brazil | Case series | 4 | M:F, 1:3 | 68.75 (57–84) | - | Hypertension, hypothyroidism, rectal tumor (case 2); hypertension, hypothyroidism (case 3); bipolar disorder (case 4) | - | Low |
| 74 | Emelyanova et al. [78] | 2021 | Ukraine | Case report | 1 | F | 38 | - | - | - | Low |
| 75 | Fathi et al. [79] | 2021 | Iran | Case report | 1 | F | 22 | April 2020 | - | Hospitalization (2nd day) | Low |
| 76 | Shenoy et al. [80] | 2022 | India | Case report | 1 | F | 55 | - | - | - | Low |
| 77 | Palaia et al. [81] | 2022 | Italy | Case report | 1 | F | 30 | - | - | - | Low |
| 78 | Rafalowicz et al. [82] | 2022 | Poland | Case series | 6 | M, 4; F, 2 | 58.8 (43–72) | January–June 2021 | Hypertension, insulin resistance (case 2) | No | Low |
| 79 | Jogdand et al. [83] | 2023 | India | Case series | 2 | M, 1; F, 1 | 50 and 60 | June 2020 | Diabetes, hypertension (case 1) | No | Low |
| No. | Study | Year of publication | Presenting symptoms given by the patient | Site | Oral signs and symptoms | Occurrence/duration of oral manifestation | Systemic manifestation |
|---|---|---|---|---|---|---|---|
| 1 | Nuno-Gonzalez et al. [5] | 2021 | Oral mucosal changes (11.7%), transient anterior U-shaped lingual papillitis (11.5%), tongue swelling (6.6%), aphthous stomatitis (6.9%), burning sensation in the mouth (5.3%), mucositis (3.9%), glossitis with patchy depapillation (3.9%), white tongue (1.6%), and enanthema (0.5%), taste disturbances | Tongue, oral mucosa | Redness and burning sensation, oral ulceration, red and white lesions, morphological changes of tongue, taste alteration | - | - |
| 2 | Sinadinos and Shelswell [6] | 2020 | Pain in palate (case 1); pain and ulcerations in palate (case 2), pain in tongue, blisters of the labial mucosa; desquamative gingivitis (case 3) | Palate, tongue, gums, lips | Oral ulceration, vesiculobullous lesions, redness and burning sensation, gingival and periodontal changes | - | Sore throat (case 1), pneumonia (case 3) |
| 3 | Dima et al. [8] | 2020 | Oral candidiasis | Oral mucosa | Red and white lesions/fungal | - | Epistaxis and diaper erythema (all 3 cases); palpebral edema (newborn 2) |
| 4 | Amorim Dos Santos et al. [9] | 2020 | Hypogeusia, white plaque, multiple pinpoint yellowish ulcers in the tongue, nodule in lower lip (1 cm) | Tongue, lower lip | Vesiculobullous lesions, red and white lesions, taste alteration | Mean duration, 14 d | Respiratory symptoms and progressive dyspnea on exertion, fever and diarrhea |
| 5 | Al-Zaidi and Badr [12] | 2020 | Loss of taste (83%) | Tongue | Taste alteration | 1 wk before systemic symptoms | Fever (63.08%), cough (60.00%), dyspnea (47.69%), sore throat, diarrhea (32.31%), chest pain (30.77%) |
| 6 | Biadsee et al. [13] | 2020 | Taste alteration (n=67), dry mouth (n=72), plaque-like changes in the tongue (n=9), swelling in the oral cavity (n=10) | Tongue, oral mucosa | Red and white lesions, taste alteration, xerostomia | Along with systemic symptoms | Cough and runny nose (p=0.018), olfactory dysfunction |
| 7 | Bodnia and Katzenstein [14] | 2020 | Total loss of taste (70%) | Tongue | Taste alteration | 1–3 wk (78%), 3–6 wk (22%) | Fatigue, headache, fever, dry cough, disturbance of the sense of smell |
| 8 | Chen et al. [15] | 2020 | Amblygeustia (47.2%), dry mouth (11.1%) | Tongue, oral mucosa | Taste alteration, xerostomia | Along with systemic symptoms | Submandibular lymph node enlargement (n=1), cough (n=21), fever (n=20), diarrhea (n=4), chest tightness (n=13) |
| 9 | Dell’Era et al. [16] | 2020 | Taste disorders (65.5%) | Tongue | Taste alteration | Mean duration, 10 d | Fever (72.1%), cough (47.9%), fatigue (40.3%), dyspnea (21.7%), diarrhea (19.7%) |
| 10 | Kumar et al. [17] | 2021 | Taste, dysfunction (28.4%) | Tongue | Taste alteration | Duration, 2–15 d | Malaise (14.2%), sore throat (19.9%), cough (20.6%), fever (48.2%), diarrhea (5.7%), nasal discharge (3.5%), headache (5.7%) |
| 11 | Lechien et al. [18] | 2020 | Gustatory dysfunction (88.8%) | Tongue | Taste alteration | Mean duration, 9.2±6.2 d | Olfactory dysfunction (85.6%) |
| 12 | Paderno et al. [19] | 2020 | Gustatory dysfunction (group A, 51.9%; group B, 78.9%); partial, 36.8%; total, 60.1%; unable to assess, 3.1% | Tongue | Taste alteration | First symptom in 11.9% (group A) and 10.2% (group B); mean duration, 9.2±5.4 | Olfactory dysfunction, fever, cough, headache, dyspnea, asthenia, diarrhea, nausea, nasal congestion, pharyngodynia |
| 13 | Boscolo-Rizzo et al. [20] | 2020 | Loss of taste (n=113) | Tongue | Taste alteration | Mean duration, 9.5 d | Dry cough, fever, headache, sore throat, chest pain, nausea, abdominal pain |
| 14 | Yan et al. [21] | 2020 | Gustatory impairment (71%) (p<0.001) | Tongue | Taste alteration | - | Fatigue (81%), fever (70%), anosmia (68%), myalgia or arthralgia (63%), diarrhea (48%), nausea (27%) |
| 15 | Sinjari et al. [22] | 2020 | Impaired taste (25%), burning sensation (15%), difficulty in swallowing (20%), dry mouth (30%) (p=0.02) | Oral mucosa, tongue | Redness and burning sensation, taste alteration, xerostomia | - | - |
| 16 | Giacomelli et al. [23] | 2020 | Dysgeusia (8.5%), ageusia (1.7%) | Tongue | Taste alteration | Before hospitalization (91%) | Fever (72.8%), cough (37.3%), dyspnea (25.4%), sore throat (1.7%), arthralgia (5.1%), headache (3.4%), asthenia (1.7%), abdominal symptoms (8.5%) |
| 17 | Mascitti et al. [24] | 2020 | Oral lichenoid reaction (32.5%), oral enanthema (27.5%), macroglossia (25.0%), cheilitis (12.5%), ageusia (20.5%), extensive ulcerations of the tongue (2.5%) | Lips, tongue, oral mucosa | Oral ulceration, vesiculobullous lesions. taste alteration | - | Macular exanthema (80%), face edema (32%), livedo (13%), urticarial rash (8%), purpura (5%), oral lichenoid lesions (33%), conjunctivitis (18%) |
| 18 | Salehi et al. [25] | 2020 | White plaques on the intraoral mucous layer of oral mucosa | Oral mucosa | Red and white lesions | - | - |
| 19 | Askin et al. [26] | 2020 | Aphthous stomatitis (5.8%), rash and erythema, aphthous lesion on side of tongue | Tongue, oral mucosa | Vesiculobullous lesions, redness and burning sensation | - | Cutaneous findings (36.1%) |
| 20 | Katz and Yue [27] | 2021 | Recurrent aphthous stomatitis (0.64%) | Oral mucosa | Oral ulceration | - | - |
| 21 | Fantozzi et al. [28] | 2020 | Dry mouth (45.9%), swallowing difficulties (39.2%), dysgeusia (59.5%) | Tongue, oral mucosa | Redness and burning sensation, oral ulceration, taste alteration, xerostomia | First symptom (xerostomia) (19.6%); dysgeusia (87.9%), duration (xerostomia), 7 d; dysgeusia 6 d | Fever (90.9%), cough (46.8%), dyspnea (34.3%), diarrhea (4.5%), sore throat (3.6%), fatigue (3.6%), myalgia/arthralgia (2.7%), vomiting (2.7%) |
| 22 | Favia et al. [29] | 2021 | Geographic tongue (n=7), fissured tongue (n=5), ulcerative lesion (n=65), blisters (n=19), hyperplasia of papillae (n=48), angina bullosa (n=11), candidiasis (n=28), ulceronecrotic gingivitis (n=7), petechiae (n=14), oral haemorrhage (n=1), taste disorders (90%) | Tongue, oral mucosa, lips | Red and white lesions, morphological changes of tongue, vesiculobullous lesions, oral ulceration, taste alteration | Together with general symptoms (26.2%); duration, 1 wk (41%); after 1 wk of general symptoms (32.6%) | Fever, anosmia, cough, sore throat, congestion, runny nose, nausea or vomiting, muscle and body aches, dermatologic manifestation, pneumonia, dyspnea, hypoxia (SpO2 <90%) |
| 23 | Halepas et al. 2021 [30] | 2021 | Red and/or swollen lips (48.9%), strawberry tongue (10.6%) | Lips, tongue | Redness and burning sensation, morphological changes of tongue | - | Fever |
| 24 | Rekhtman et al. [31] | 2021 | Rashes on lips and tongue (5.7% and 2.9%), ulcers on lips and tongue | Lips and tongue | Redness and burning sensation, oral ulceration | Generalized rashes | |
| 25 | Marouf et al. [32] | 2021 | Periodontitis (258/568) | Periodontium | Gingival and periodontal changes | - | - |
| 26 | Subramaniam et al. [33] | 2021 | Ulcers on oral mucosa; burning mouth and mucositis on lower labial mucosa, papillary atrophy; reddish-white spots on the palate; ulcers on lower lip; pallor of lip | Oral mucosa palate, lips, tongue | Oral ulceration, petechiae, redness and burning sensation, morphological changes of tongue | - | Fever, cough, dyspnea, runny nose, chest tightness, loss of smell |
| 27 | Abubakr et al. [34] | 2021 | Dental pain (23%), pain in jaw bones or joint (12.0%), halitosis (10.5%), ulcerations (20.4%), dry mouth (47.6%) | Oral mucosa | Oral ulceration, redness and burning sensation, xerostomia | - | Fever, myalgia, dysphagia, and hyposmia, loss of smell, nasal itching |
| 28 | Song et al. [35] | 2021 | Loss of taste (20.6%) | Tongue | Taste alteration | First symptom (0.4%), recovery time, 7 d | Nasal obstruction (8.6%), rhinorrhea (10.3%), nasal itching (4.9%), sneezing (11.0%), loss of smell (11.4%) |
| 29 | Bardellini et al. [36] | 2021 | Oral pseudomembranous candidiasis (7.4%), geographic tongue (3.7%), coated tongue (7.4%), taste alteration (11.1%) | Tongue, oral mucosa | Red and white lesions, morphological changes of tongue, taste alteration | - | Fever, cough, rhinorrhoea, breathing difficulty |
| 30 | Gherlone et al. [37] | 2021 | Salivary gland ectasia (38%), dry mouth (30%), dysgeusia (17%), white plaque (28%), oral ulcers (12%) | Salivary glands, tongue, oral mucosa | Red and white lesions, oral ulceration, xerostomia, salivary gland disorder | - | - |
| 31 | El Kady et al. [38] | 2021 | Dry mouth (39.7%), loss of salt sensation (34.5%), loss of sweet sensation (29.3%), altered food taste (25.9%), tongue redness (8.8%), gingival bleeding (7%), salivary glands infection (22.4%), swellings in the salivary gland or cheek (13.8%), pain or swelling below mandible (10.8%), burning mouth sensation (22.4%), ulcers (17.2%) | Tongue, salivary glands, gingiva, oral mucosa | Redness and burning sensation, taste alteration, xerostomia, gingival and periodontal changes, salivary gland disorder | - | - |
| 32 | Fidan et al. [39] | 2021 | Aphthous-like ulcer (36.5%), erythema (25.7%), lichen planus (16.2%), tongue (31.8%), oral mucosa (27.0%), gingiva (18.9%), palate (5.4%) | Tongue (39.7%), oral mucosa (34.5%), gingiva (18.9%), palate (6.9%) | Oral ulceration, redness and burning sensation, red and white lesions | Oral lesions prior COVID-19 diagnosis | - |
| 33 | Natto et al. [40] | 2021 | Loss of taste (43.4%), erythema/desquamated gingivitis and coated tongue (7.3%), ulcers/blisters (6.4%), pain and soreness (2.8%), dry mouth (0.9%) | Tongue, gingiva, oral mucosa | Oral ulceration, redness and burning sensation, taste alteration, xerostomia, gingival changes | After systemic symptoms | Cough, fever, sore throat, runny nose, muscle pain, headaches, nausea, diarrhea |
| 34 | Elamrousy et al. [41] | 2021 | Oral ulcers (92.8%), dry mouth (84%), loss of taste (55%), hemorrhagic ulcers with crust on lips | Lip (42.3%), tongue (38.5%), labial mucosa (34.6%) | Oral ulceration, redness and burning sensation, taste alteration, xerostomia | - | Asthenia (67.7%), breath problems (67.7%), cough (67.7%), fatigue (19.4%), abdominal symptoms (12.9%) |
| 35 | Bulut et al. [42] | 2021 | Taste loss (53%), halitosis (21%), oropharyngeal wound and pain (18%), pain in the chewing muscles (16%), gum bleeding (17.5%), dry mouth (38%, after recovery 12.0%), aphthous ulcer (14.5%), sensitivity and/or pain in teeth (12%), herpes labialis (8.5%), burning in the tongue (7.5%) | Tongue, gingiva, lips | Oral ulceration, redness and burning sensation, taste alteration, xerostomia | - | Presence of symptoms (87.5%) |
| 36 | Naser et al. [43] | 2021 | Burning sensation (6%), numbness or tingling of the tongue (2%), white coat of the tongue, gingiva, palate (31.6%, 22.4%, 15.6%), loss of taste (79.5%), aphthous ulcers (24.8%), black discoloration of oral cavity, lips and tongue (4.7%, 6.8%), yellow coating on lips (5.3%) | Tongue, palate, lips, oral mucosa | Oral ulceration, redness and burning sensation, red and white lesions, taste alteration | - | - |
| 37 | Muthyam et al. [44] | 2022 | Dry mouth (44%) followed by swallowing difficulty, mouth ulcerations, chewing problems, gum bleeding, and burning sensation, altered taste (72%); fissured tongue, halitosis, and loss of taste, 2% | Gums, tongue, oral mucosa | Oral ulceration, redness and burning sensation, morphological changes of tongue, taste alteration, xerostomia | Altered taste lasted more than 1 wk (53%) | Weakness (8%), cough and cold (4%), body pain (2%) |
| 38 | Ganesan et al. [45] | 2022 | Gustatory disturbance (51.2%); dry mouth (28%); erythema, ulcers and depapillation of tongue (15.5%); A statistically significant correlation between oral manifestations and disease severity (p≤0.001). | Tongue, oral mucosa | Morphological changes of tongue, oral ulceration, taste alteration, xerostomia | - | - |
| 39 | El Tantawi et al. [46] | 2022 | Dry mouth (11.1% vs. 7.5%, p=0.009) and change in taste (11.5% vs. 2.7%, p<0.001) were greater in COVID-19 person; leukoplakia (4.6%); ulcers & hairy tongue (2.3%); gingival redness and burning sensation (13.1%) | Oral mucosa tongue, gingiva | Morphological changes of tongue, oral ulceration, redness and burning sensation, taste alteration, xerostomia, gingival and periodontal changes | - | - |
| 40 | Soares et al. [47] | 2022 | Ulcerative lesions in the palate (57.1%), tongue (29%), lips or palate (14.3%) | Tongue, lips, palate | Oral ulceration | - | Anosmia, fever, headache |
| 41 | Tuter et al. [48] | 2022 | Dry mouth (44.2%), oral ulceration (22.4%), oral mucosa (15.2%), tongue (10.8%) | Tongue, oral mucosa | Oral ulceration, redness and burning sensation | - | - |
| 42 | Schwab et al. [49] | 2022 | Ageusia (11.0%); opportunistic oral infections such as pseudomembranous candidiasis and herpes simplex (4.5%) | Tongue | Red and white lesions/fungal, taste alteration, oral ulceration | - | Cough (72.7%), dyspnoea (63.0%), fever (53.9%), anosmia (14.3%) |
| 43 | Chawla et al. [50] | 2022 | Dry mouth (38%) (p=0.03), dysgeusia (32%) (p=0.04), vesiculobullous lesion (13%), oral ulcers (3.7%) | Oral mucosa tongue | Oral ulceration, redness and burning sensation, vesiculobullous lesions, taste alteration, xerostomia | - | Cough (30%), sore throat (20%), shortness of breath (7%), running nose (11%) |
| 44 | Binmadi et al. [51] | 2022 | Taste disturbance (60%); dry mouth (42%); oral ulcerations (11%); gingivitis/petechiae/candidiasis (6%); necrotizing periodontal disease/vesiculobullous lesions/erythema migrans/geographic tongue (4%) | Gingiva, tongue, oral mucosa | Oral ulceration, redness and burning sensation, vesiculobullous lesions, morphological changes of tongue, red and white lesions, taste alteration, xerostomia | Concurrently (47%), after the general symptoms (43%), before the general symptoms (9%) | Fever (95%), headache (65%), fatigue (65%), cough (63%), myalgia/arthralgia (53%), loss of smell (53%), sore throat (50%), shortness of breath or dyspnea (40%), nausea or vomiting (21%), diarrhea (15%) |
| 45 | Eduardo et al. [52] | 2022 | Saliva alterations (24.4%), dryness (9.9%), tongue coating (3%), sialorrhea (3.3%), petechiae (10.5%), oral bleeding (7.5%) | Oral mucosa tongue, salivary glands | Red and white lesions, redness and burning sensation, salivary gland disorder, xerostomia | - | - |
| 46 | Villarroel-Dorrego et al. [53] | 2022 | Hemorrhagic ulcerative lesions (7.3%), erythematous and pseudomembranous forms of candidiasis (12.7%), angular cheilitis (1.5%), total loss of taste (60%), burning mouth (36.4%), dry mouth (27.3%) | Tongue, lips, oral mucosa | Oral ulceration, redness and burning sensation, red and white, lesions, xerostomia | - | - |
| 47 | Manifar et al. [54] | 2022 | Dry mouth (68.6%) (p<0.001), dysgeusia (51.4%) (p<0.001), hypogeusia (49.3%), halitosis (31.4%), metallic taste (29.3%) | Tongue, oral mucosa | Redness and burning sensation, taste alteration, xerostomia | - | Gastrointestinal symptoms, smell defects, asthma, skin rashes, cough, malaise, myalgia, anorexia, respiratory distress, olfactory dysfunction |
| 48 | Bhuyan et al. [55] | 2022 | Burning sensation (2.4%), dry mouth (2.4%), loss of taste (31%) (p<0.001), mouth ulcer (2.4%), bleeding gum (2.4%) | Oral mucosa gums, tongue | Oral ulceration, redness and burning sensation, taste alteration, xerostomia, gingival changes | - | - |
| 49 | Brandao et al. [56] | 2021 | Multiple aphthous-like ulcers covered with mucopurulent membrane in the upper and lower lip mucosa and tongue (cases 1, 2, 4, 5); ulcers on tongue and hard palate (case 3); ulcers on tongue and ageusia (cases 6, 7, 8) | Lips, tongue, palate | Oral ulceration, taste alteration | 6–10 d | Chest tightness, fever, cough (cases 1, 5, 7, 8); cough, fever, dyspnea (cases 2, 6); abdominal distension, fever, mild dyspnea (cases 3, 4) |
| 50 | Cruz Tapia et al. [57] | 2020 | Bulla on the hard palate (×6 mm) (case 1); diffuse purple macule (×12 mm) and papule-plaque (×8 mm) on the left and right palatal mucosa (case 2); tongue enlargement (case 3); Burning mouth sensation and reddish macules on hard palate (case 4) | Palate, tongue | Redness and burning sensation, vesiculobullous lesions, morphological changes of tongue | Fever, myalgia, dysphagia, hyposmia | |
| 51 | Vaira et al. [58] | 2020 | Hypogeusia (33 cases); complete ageusia (1 case) | Tongue | Taste alteration | Fever, cough, nasal obstruction, sore throat, hyposmia, anosmia, pneumonia | |
| 52 | Martin Carreras-Presas et al. [59] | 2021 | Dysgeusia (case 1); multiple ulcers | Tongue, lips | Oral ulceration, redness and burning sensation, vesiculobullous lesions, taste alteration, gingival changes | Along with systemic symptoms | Asthenia, hyposmia, enlargement of lymph nodes in the neck (cases 1, 3); fever, diarrhea (case 2) |
| On palate (case 2); pain on tongue, blisters in lip mucosa and | |||||||
| Desquamative gingivitis (case 3) | |||||||
| 53 | Rodriguez et al. [60] | 2022 | Dysgeusia, aphthous‐like lesions, burning sensation, and tongue depapillation (case 1); burning mouth sensation and unilateral commissural fissures (case 2); dry mouth, lesions on the tongue, palate, and commissure (case 3) | Tongue, palate, oral mucosa | Redness and burning sensation, morphological changes of tongue, taste alteration, xerostomia | Before presentation (case 1); after discharge (case 2); with systemic symptoms (case 3) | Fever, malaise, anosmia, diarrhea, pneumonia (cases 1, 3) |
| 54 | Corchuelo and Ulloa [61] | 2020 | Reddish plaques on the lower lip, dark brown pigmentation and aphthous ulcers in the gums, whitish area in tongue | Lower lips, gums | Oral ulceration, vesiculobullous lesions, red and white lesions | Mean duration, 8–10 d | - |
| 55 | Eghbali Zarch and Hosseinzadeh [62] | 2021 | Vesicles on lower lip mucosa | Lip | Vesiculobullous lesions | 2 d before systemic symptoms | High fever, fatigue, lack of appetite |
| 56 | Hjelmesaeth and Skaare [63] | 2020 | Total ageusia | Tongue | Taste alteration | - | - |
| 57 | Cebeci Kahraman and Caskurlu [64] | 2020 | Erythematous surface (hard palate), few petechiae in the midline and numerous pustular enanthema near the soft palate border | Palate | Red and white lesions, oral ulceration | Mean duration, 10 d | Sore throat; fever, fatigue, severe dry cough, inability to taste or smell |
| 58 | Smith et al. [65] | 2020 | Loss of taste | Tongue | Taste alteration | Before general symptoms | Frontal headache, loss of smell, headache, loose stools |
| 59 | Maniaci et al. [66] | 2020 | Transient loss of taste | Tongue | Taste alteration | Mean duration, 12 d | Fever, sore throat, runny nose, presence of erythematous skin lesions on the lower limbs, asthenia |
| 60 | Melley et al. [67] | 2020 | Loss of taste | Tongue | Taste alteration | 1 wk before systemic presentation | Shortness of breath, fatigue, loss of appetite |
| 61 | Riad et al. [68] | 2022 | Painful white patches on the dorsal surface of the tongue and palate, mild tongue pain | Tongue, palate | Red and white lesions | 2 wk before diagnosis | Sore throat, generalized myalgia, fatigue with intermittent fever |
| 62 | Putra et al. [69] | 2020 | Stomatitis aphthous | Oral mucosa | Oral ulceration, redness and burning sensation | Day 7 | Fever, back pain, myalgia, sore throat, dry cough, rhinorrhea, anosmia |
| 63 | Dalipi et al. [70] | 2021 | Loss of taste | Tongue, lips | Vesiculobullous lesions, taste alteration | Loss of taste, 2 wk before diagnosis | Fever, cough, headache, muscle pain, loss of smell, dark red, purpuric, irregular maculopapular lesions on abdomen |
| Bullous and erosive erythematous lesions of lips and oral mucosa | |||||||
| 64 | Eita [71] | 2021 | Dysgeusia and greasy tongue coat | Tongue | Morphological changes of tongue, taste alteration | Before systemic symptoms | Sore throat, fever (38 °C), nasal congestion, conjunctivitis, nausea; abdominal pain, diarrhea, fatigue, severe joint pain |
| 65 | Cirillo and Colella [72] | 2021 | Loss of taste | Tongue | Taste alteration | 1 wk before presentation | Loss of smell, headache, fatigue, muscle pain |
| 66 | Nejabi et al. [73] | 2021 | Fissured tongue, white scars and painful erosive ulcer on the dorsal surface of the tongue | Tongue | Morphological changes of tongue, oral ulceration | After 1 wk of general symptoms | Fever, cough, taste alterations, olfactory dysfunction, chest tightness |
| 67 | Klein et al. [74] | 2021 | Loss of taste | Tongue | Taste alteration | From 4th to 6 wk | Fever, dry cough, chest pain, sore throat, diarrhea, nausea, headache, back pain |
| 68 | Ramires et al. [75] | 2021 | Crusted ulcers on lip vermilion (both upper and lower lips) | Lip | Oral ulceration | 2 wk after the onset of fever | Flu-like syndrome: severe and progressive dyspnea (SpO2=88%) |
| 69 | Hockova et al. [76] | 2021 | Oral lesions at the dorsal surface of the tongue (case 1); multiple lesions located on the tongue dorsum and labial mucosa (case 2); lesions on upper and lower lip (case 3) | Tongue, lips | Oral ulceration | After the diagnosis (all 3 cases) | Headache, fever, dry cough, dyspnoea |
| 70 | Teixeira et al. [77] | 2021 | Painful vesiculobullous lip lesions | Lips | Vesiculobullous lesions | After 4 d (case 1); after 10 d (case 2); after 11 d (case 3); after 19 d (case 4) | Headache, myalgia, dyspnea |
| 71 | Emelyanova et al. [78] | 2021 | Unusual tongue appearance and burning sensation, intermittent bleeding of gums, severe dryness in the oral cavity and persistent distortion of taste | Tongue, gums, oral mucosa | Redness and burning sensation, morphological changes of tongue, xerostomia | 3rd day (dysgeusia) and 5th day (xerostomia) after systemic symptoms | Rhinorrhea, coughing and increased body temperature to 38.5 °C |
| 72 | Fathi et al. [79] | 2021 | Oral pain, ulcerative lesions on oral mucosa, hemorrhagic crusts on lips | Oral mucosa, lips | Oral ulceration, redness and burning sensation | 3rd day (oral pain) | Fever, abdominal pain, nausea, occasional vomiting |
| 73 | Shenoy et al. [80] | 2022 | Ulcer with irregular borders on the dorsum of the tongue surrounded by a scrapable whitish plaque | Tongue | Oral ulceration, red and white lesions | Systemic symptoms, 3 wk prior | Fever, cough, chest tightness |
| 74 | Palaia et al. [81] | 2022 | Extensive erosions involving lips, ulcers on the hard palate, blisters and ulcers on the dorsal surface of the tongue cheek mucosa | Palate, lips, oral mucosa | Oral ulceration, vesiculobullous lesions, redness and burning sensation | 7 d prior to general symptoms (duration of oral lesions, 14 d) | Bilateral cutaneous lesions were also evident on the hands. Low-grade fever |
| 75 | Rafalowiczet al. [82] | 2022 | Unilateral aphthous-like lesions on the left side of the hard palate (case 1 & 5); hemorrhagic changes on the palate and cheilitis (case 2); smooth tongue with intensely red-purple mucosa (case 3); angioma-type lesion on the right side of the palate (case 4); mycosis of the tongue, extensive lesions on the palate, cheilitis (case 6) | Hard palate, tongue, lips | Oral ulceration, redness and burning sensation, red and white/fungal, morphological changes of tongue | - | Fever, malaise, taste disorders, anosmia, and pneumonia (case 1); dyspnea, persistent diarrhea, and vomiting (case 2); loss of smell and taste and fever for 9 d (cases 4, 5) |
| 76 | Jogdand et al. [83] | 2023 | Ulcers with yellowish gray pseudo-membrane on oral mucosa and palate | Oral mucosa, palate | Oral ulceration, red and white lesions | - | - |
| 77 | Mohammad et al. [84] | 2023 | Dry mouth (50%), gustatory dysfunction (37%), burning mouth sensation (22.5%), oral pain (17%), aphthous lesions, fissural cheilitis and tongue depapillation (9.5%), candidiasis (7.5%), gingival bleeding (2.5%) | Gingiva, oral mucosa, tongue | Oral ulceration, red and white lesions, morphological changes of tongue, taste alteration, xerostomia, gingival changes | - | Fever (83.5%), weakness (80%), myalgia (73%), headache (70%), cough (65%), loss of smell sensation (54%), loss of taste sensation (48.5%), sore throat (38.5%), nasal congestion (26.5%), runny nose (25%), gastrointestinal symptom (24.5%) |
| 78 | Al-Magsoosi et al. [85] | 2023 | Ageusia (66.8%), dry mouth (59%), gustatory changes (46%), dysphagia (40.5%), burning sensation (20.8%), oral ulceration (14.5%), gingival bleeding (3.3%) | Tongue, oral mucosa, gingiva | Redness and burning sensation, taste alteration, xerostomia | - | - |
| 79 | Cazzolla et al. [86] | 2023 | Taste dysfunction (208/25%) | Tongue | Taste alteration | 1 wk before general symptoms | Fever, breathing, asthenia, rhinorrhea, headache, abdominal symptoms, sore throat, chest pain, cough |
- 1. World Health Organization (WHO). WHO coronavirus (COVID-19) dashboard [Internet]. WHO; 2023 [cited 2023 Apr 9]. Available from: https://covid19.who.int/.
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708−20.ArticlePubMed
- 3. Kipshidze N, Dangas G, White CJ, et al. Viral coagulopathy in patients with COVID-19: treatment and care. Clin Appl Thromb Hemost 2020;26:1076029620936776. ArticlePubMedPMCPDF
- 4. Amorim Dos Santos J, Normando AG, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID-19: a living systematic review. J Dent Res 2021;100:141−54.ArticlePubMedPDF
- 5. Nuno-Gonzalez A, Martin-Carrillo P, Magaletsky K, et al. Prevalence of mucocutaneous manifestations in 666 patients with COVID-19 in a field hospital in Spain: oral and palmoplantar findings. Br J Dermatol 2021;184:184−5.ArticlePubMedPMCPDF
- 6. Sinadinos A, Shelswell J. Oral ulceration and blistering in patients with COVID-19. Evid Based Dent 2020;21:49. ArticlePubMedPMCPDF
- 7. de Sousa FA, Paradella TC. Considerations on oral manifestations of COVID-19. J Med Virol 2021;93:667−8.ArticlePubMedPDF
- 8. Dima M, Enatescu I, Craina M, et al. First neonates with severe acute respiratory syndrome coronavirus 2 infection in Romania: three case reports. Medicine (Baltimore) 2020;99:e21284.PubMedPMC
- 9. Amorim Dos Santos J, Normando AG, Carvalho da Silva RL, et al. Oral mucosal lesions in a COVID-19 patient: new signs or secondary manifestations? Int J Infect Dis 2020;97:326−8.ArticlePubMedPMC
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. ArticlePubMedPMC
- 11. Moola S, Munn Z, Tufanaru C, et al. Chapter 7: systematic reviews of etiology and risk [Internet]. Edited by Aromataris E, Munn Z: JBI manual for evidence synthesis. Joanna Briggs Institute; 2020. [cited 2023 Apr 9]. Available from: https://synthesismanual.jbi.global.
- 12. Al-Zaidi HM, Badr HM. Incidence and recovery of smell and taste dysfunction in COVID-19 positive patients. Egypt J Otolaryngol 2020;36:47. ArticlePDF
- 13. Biadsee A, Biadsee A, Kassem F, et al. Olfactory and oral manifestations of COVID-19: sex-related symptoms: a potential pathway to early diagnosis. Otolaryngol Head Neck Surg 2020;163:722−8.ArticlePubMedPMCPDF
- 14. Bodnia NC, Katzenstein TL. Acute loss of smell and taste among patients with symptoms compatible with COVID-19. Dan Med J 2020;67:A05200370. PubMed
- 15. Chen L, Zhao J, Peng J, et al. Detection of SARS-CoV-2 in saliva and characterization of oral symptoms in COVID-19 patients. Cell Prolif 2020;53:e12923.ArticlePubMedPMCPDF
- 16. Dell’Era V, Farri F, Garzaro G, et al. Smell and taste disorders during COVID-19 outbreak: cross-sectional study on 355 patients. Head Neck 2020;42:1591−6.PubMedPMC
- 17. Kumar L, Kahlon N, Jain A, et al. Loss of smell and taste in COVID-19 infection in adolescents. Int J Pediatr Otorhinolaryngol 2021;142:110626. ArticlePubMedPMC
- 18. Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251−61.ArticlePubMedPMCPDF
- 19. Paderno A, Schreiber A, Grammatica A, et al. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol 2020;10:955−62.ArticlePubMedPMCPDF
- 20. Boscolo-Rizzo P, Borsetto D, Fabbris C, et al. Evolution of altered sense of smell or taste in patients with mildly symptomatic COVID-19. JAMA Otolaryngol Head Neck Surg 2020;146:729−32.ArticlePubMedPMC
- 21. Yan CH, Faraji F, Prajapati DP, et al. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol 2020;10:806−13.ArticlePubMedPMCPDF
- 22. Sinjari B, D’Ardes D, Santilli M, et al. SARS-CoV-2 and oral manifestation: an observational, human study. J Clin Med 2020;9:3218. ArticlePubMedPMC
- 23. Giacomelli A, Pezzati L, Conti F, et al. Self-reported olfactory and taste disorders in patients with severe acute respiratory coronavirus 2 infection: a cross-sectional study. Clin Infect Dis 2020;71:889−90.ArticlePubMedPDF
- 24. Mascitti H, Bonsang B, Dinh A, et al. Clinical cutaneous features of patients infected with SARS-CoV-2 hospitalized for pneumonia: a cross-sectional study. Open Forum Infect Dis 2020;7:ofaa394. ArticlePubMedPMCPDF
- 25. Salehi M, Ahmadikia K, Mahmoudi S, et al. Oropharyngeal candidiasis in hospitalised COVID-19 patients from Iran: species identification and antifungal susceptibility pattern. Mycoses 2020;63:771−8.ArticlePubMedPMCPDF
- 26. Askin O, Altunkalem RN, Altinisik DD, et al. Cutaneous manifestations in hospitalized patients diagnosed as COVID-19. Dermatol Ther 2020;33:e13896.PubMedPMC
- 27. Katz J, Yue S. Increased odds ratio for COVID-19 in patients with recurrent aphthous stomatitis. J Oral Pathol Med 2021;50:114−7.ArticlePubMedPDF
- 28. Fantozzi PJ, Pampena E, Di Vanna D, et al. Xerostomia, gustatory and olfactory dysfunctions in patients with COVID-19. Am J Otolaryngol 2020;41:102721. ArticlePubMedPMC
- 29. Favia G, Tempesta A, Barile G, et al. COVID-19 symptomatic patients with oral lesions: clinical and histopathological study on 123 cases of the University Hospital Policlinic of Bari with a purpose of a new classification. J Clin Med 2021;10:757. ArticlePubMedPMC
- 30. Halepas S, Lee KC, Myers A, et al. Oral manifestations of COVID-2019-related multisystem inflammatory syndrome in children: a review of 47 pediatric patients. J Am Dent Assoc 2021;152:202−8.ArticlePubMed
- 31. Rekhtman S, Tannenbaum R, Strunk A, et al. Eruptions and related clinical course among 296 hospitalized adults with confirmed COVID-19. J Am Acad Dermatol 2021;84:946−52.ArticlePubMed
- 32. Marouf N, Cai W, Said KN, et al. Association between periodontitis and severity of COVID-19 infection: a case-control study. J Clin Periodontol 2021;48:483−91.ArticlePubMedPMCPDF
- 33. Subramaniam T, Nikalje MR, Jadhav S. Oral manifestations among COVID-19: an observational study of 713 patients. Dent Res J (Isfahan) 2021;18:67. ArticlePubMedPMC
- 34. Abubakr N, Salem ZA, Kamel AH. Oral manifestations in mild-to-moderate cases of COVID-19 viral infection in the adult population. Dent Med Probl 2021;58:7−15.ArticlePubMed
- 35. Song J, Deng YK, Wang H, et al. Self-reported taste and smell disorders in patients with COVID-19: distinct features in China. Curr Med Sci 2021;41:14−23.ArticlePubMedPMCPDF
- 36. Bardellini E, Bondioni MP, Amadori F, et al. Non-specific oral and cutaneous manifestations of coronavirus disease 2019 in children. Med Oral Patol Oral Cir Bucal 2021;26:e549−53.ArticlePubMedPMC
- 37. Gherlone EF, Polizzi E, Tete G, et al. Frequent and persistent salivary gland ectasia and oral disease after COVID-19. J Dent Res 2021;100:464−71.ArticlePubMedPDF
- 38. El Kady DM, Gomaa EA, Abdella WS, et al. Oral manifestations of COVID-19 patients: an online survey of the Egyptian population. Clin Exp Dent Res 2021;7:852−60.ArticlePubMedPMCPDF
- 39. Fidan V, Koyuncu H, Akin O. Oral lesions in COVID 19 positive patients. Am J Otolaryngol 2021;42:102905. ArticlePubMedPMC
- 40. Natto ZS, Afeef M, Khalil D, et al. Characteristics of oral manifestations in symptomatic non-hospitalized COVID-19 patients: a cross-sectional study on a sample of the Saudi population. Int J Gen Med 2021;14:9547−53.ArticlePubMedPMCPDF
- 41. Elamrousy WA, Nassar M, Issa DR. Prevalence of oral lesions in COVID-19 Egyptian patients. J Int Soc Prev Community Dent 2021;11:712−20.ArticlePubMedPMC
- 42. Bulut DG, Turker N, Serin S, et al. The effect of severe acute respiratory syndrome coronavirus 2 on the health of oral tissue: a survey-based study. J Oral Health Oral Epidemiol 2021;10(Special Issue):43−9.
- 43. Naser AI, Al Sarraj MN, Deleme ZH. Oral and maxillofacial lesions in COVID 19 infection from Mosul hospital in Iraq: epidemiological study and approach to classification and treatment. J Oral Res 2021;10:1−14.Article
- 44. Muthyam AK, Reddy MP, Kulkarni S, et al. Oral manifestations in COVID-19 patients: an observational study. J Family Med Prim Care 2022;11:1000−5.ArticlePubMedPMC
- 45. Ganesan A, Kumar S, Kaur A, et al. Oral manifestations of COVID-19 infection: an analytical cross-sectional study. J Maxillofac Oral Surg 2022;21:1326−35.ArticlePubMedPMCPDF
- 46. El Tantawi M, Sabbagh HJ, Alkhateeb NA, et al. Oral manifestations in young adults infected with COVID-19 and impact of smoking: a multi-country cross-sectional study. PeerJ 2022;10:e13555.ArticlePubMedPMCPDF
- 47. Soares CD, Souza LL, de Carvalho MG, et al. Oral manifestations of coronavirus disease 2019 (COVID-19): a comprehensive clinicopathologic and immunohistochemical study. Am J Surg Pathol 2022;46:528−36.PubMed
- 48. Tuter G, Yerebakan M, Celik B, et al. Oral manifestations in SARS-CoV-2 infection. Med Oral Patol Oral Cir Bucal 2022;27:e330−9.ArticlePubMedPMC
- 49. Schwab G, Palmieri M, Zerbinati RM, et al. Lack of direct association between oral mucosal lesions and SARS-CoV- 2 in a cohort of patients hospitalised with COVID-19. J Oral Microbiol 2022;14:2047491. ArticlePubMedPMC
- 50. Chawla J, Y N, Bakshi SS, et al. Oral manifestations associated with COVID-19 disease: an observational cross sectional study. J Oral Biol Craniofac Res 2022;12:279−83.ArticlePubMedPMC
- 51. Binmadi NO, Aljohani S, Alsharif MT, et al. Oral manifestations of COVID-19: a cross-sectional study of their prevalence and association with disease severity. J Clin Med 2022;11:4461. ArticlePubMedPMC
- 52. Eduardo FP, Bezinelli LM, Gobbi MF, et al. Oral lesions and saliva alterations of COVID-19 patients in an intensive care unit: a retrospective study. Spec Care Dentist 2022;42:494−502.ArticlePubMedPDF
- 53. Villarroel-Dorrego M, Chacon L, Rosas R, et al. Oral findings in patients with COVID-19. Actas Dermosifiliogr 2022;113:183−6. Spanish.PubMed
- 54. Manifar S, Koopaie M, Farani AK, et al. Assessment of oral manifestations and oral health in hospitalized patients with COVID-19: machine learning and statistical analysis. Ann Military Health Sci Res 2022;20:e121764.ArticlePDF
- 55. Bhuyan R, Bhuyan SK, Mohanty JN, et al. A preliminary survey on the oral manifestation of COVID-19 in the first and second waves in Bhubaneswar, City of Odisha, India. Natl J Community Med 2022;13:294−7.ArticlePDF
- 56. Brandao TB, Gueiros LA, Melo TS, et al. Oral lesions in patients with SARS-CoV-2 infection: could the oral cavity be a target organ? Oral Surg Oral Med Oral Pathol Oral Radiol 2021;131:e45−51.ArticlePubMed
- 57. Cruz Tapia RO, Peraza Labrador AJ, Guimaraes DM, et al. Oral mucosal lesions in patients with SARS-CoV-2 infection: report of four cases: are they a true sign of COVID-19 disease? Spec Care Dentist 2020;40:555−60.ArticlePubMedPDF
- 58. Vaira LA, Deiana G, Fois AG, et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck 2020;42:1252−8.PubMedPMC
- 59. Martin Carreras-Presas C, Amaro Sanchez J, Lopez-Sanchez AF, et al. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis 2021;27(Suppl 3). 710−2.ArticlePubMedPDF
- 60. Rodriguez MD, Romera AJ, Villarroel M. Oral manifestations associated with COVID‐19. Oral Dis 2022;28(Suppl 1). 960−2.ArticlePubMedPDF
- 61. Corchuelo J, Ulloa FC. Oral manifestations in a patient with a history of asymptomatic COVID-19: case report. Int J Infect Dis 2020;100:154−7.ArticlePubMedPMC
- 62. Eghbali Zarch R, Hosseinzadeh P. COVID-19 from the perspective of dentists: a case report and brief review of more than 170 cases. Dermatol Ther 2021;34:e14717.PubMedPMC
- 63. Hjelmesaeth J, Skaare D. Loss of smell or taste as the only symptom of COVID-19. Tidsskr Nor Laegeforen; 2020. p 140English, Norwegian.
- 64. Cebeci Kahraman F, Caskurlu H. Mucosal involvement in a COVID-19-positive patient: a case report. Dermatol Ther 2020;33:e13797.PubMedPMC
- 65. Smith LT, Hodges CD, Pratt M, et al. Case report: COVID-19 patient with chief complaint of anosmia and ageusia; a unique perspective on atypical symptomatology and management in the military. Mil Med 2020;185:e2176−9.ArticlePubMedPDF
- 66. Maniaci A, Iannella G, Vicini C, et al. A case of COVID-19 with late-onset rash and transient loss of taste and smell in a 15-year-old boy. Am J Case Rep 2020;21:e925813.ArticlePubMedPMC
- 67. Melley LE, Bress E, Polan E. Hypogeusia as the initial presenting symptom of COVID-19. BMJ Case Rep 2020;13:e236080.ArticlePubMedPMC
- 68. Riad A, Gad A, Hockova B, et al. Oral candidiasis in non-severe COVID-19 patients: call for antibiotic stewardship. Oral Surg 2022;15:465−6.ArticlePubMedPDF
- 69. Putra BE, Adiarto S, Dewayanti SR, et al. Viral exanthem with “spins and needles sensation” on extremities of a COVID-19 patient: a self-reported case from an Indonesian medical frontliner. Int J Infect Dis 2020;96:355−8.ArticlePubMedPMC
- 70. Dalipi ZS, Dragidella F, Dragidella DK. Oral manifestations of exudative erythema multiforme in a patient with COVID-19. Case Rep Dent 2021;2021:1148945. ArticlePubMedPMCPDF
- 71. Eita AA. Parosmia, dysgeusia, and tongue features changes in a patient with post-acute COVID-19 syndrome. Case Rep Dent 2021;2021:3788727. ArticlePubMedPMCPDF
- 72. Cirillo N, Colella G. Self-reported smell and taste alteration as the sole clinical manifestation of SARS-CoV-2 infection. Oral Surg Oral Med Oral Pathol Oral Radiol 2021;131:e95−9.ArticlePubMed
- 73. Nejabi MB, Noor NA, Raufi N, et al. Tongue ulcer in a patient with COVID-19: a case presentation. BMC Oral Health 2021;21:273. ArticlePubMedPMCPDF
- 74. Klein H, Karni N, Israel S, et al. Reversible taste loss in a COVID-19 patient with preexisting chronic smell impairment. J Investig Med High Impact Case Rep 2021;9:2324709621990765. ArticlePubMedPMCPDF
- 75. Ramires MC, Mattia MB, Tateno RY, et al. A combination of phototherapy modalities for extensive lip lesions in a patient with SARS-CoV-2 infection. Photodiagnosis Photodyn Ther 2021;33:102196. ArticlePubMedPMC
- 76. Hockova B, Riad A, Valky J, et al. Oral complications of ICU patients with COVID-19: case-series and review of two hundred ten cases. J Clin Med 2021;10:581. ArticlePubMedPMC
- 77. Teixeira IS, Leal FS, Tateno RY, et al. Photobiomodulation therapy and antimicrobial photodynamic therapy for orofacial lesions in patients with COVID-19: a case series. Photodiagnosis Photodyn Ther 2021;34:102281. ArticlePubMedPMC
- 78. Emelyanova N, Isayeva G, Komir I, et al. Changes in the oral cavity of a patient after suffering from coronavirus infection COVID-19: a clinical case. Acta Med Mediterr 2021;37:827−31.
- 79. Fathi Y, Hoseini EG, Mottaghi R. Erythema multiform-like lesions in a patient infected with SARS-CoV-2: a case report. Future Virol 2021;16:157−60.Article
- 80. Shenoy P, Archana P, Chatra L, et al. Tongue ulcer as an oral manifestation of COVID-19: a case report. Int J Res Rep Dent 2022;5:8−11.
- 81. Palaia G, Pernice E, Pergolini D, et al. Erythema multiforme as early manifestation of COVID-19: a case report. Pathogens 2022;11:654. ArticlePubMedPMC
- 82. Rafalowicz B, Wagner L, Rafalowicz J. Long COVID oral cavity symptoms based on selected clinical cases. Eur J Dent 2022;16:458−63.ArticlePubMed
- 83. Jogdand MS, Sansare KP, Karjodkar FR, et al. COVID-19-oral findings: a case report. J Clin Med Images Case Rep 2023;3:1367. Article
- 84. Mohammad NH, Hameed AY, Mahmood AN. Oro-facial manifestations of COVID-19 infection in a sample of Iraqi people. J Popul Ther Clin Pharm 2023;30:131−44.
- 85. Al-Magsoosi MJ, Al-Asadi OK, Al-Quraine NT, et al. Oral manifestations associated with COVID-19 infection: a cross-sectional study of recovered Iraqi patients. Int J Dent 2023;2023:4288182. ArticlePubMedPMCPDF
- 86. Cazzolla AP, Lovero R, Spirito F, et al. Evaluation of qualitative and quantitative taste alterations in COVID-19. Biomol Biomed 2023;23:344−50.ArticlePubMedPMCPDF
- 87. Chaux-Bodard AG, Deneuve S, Desoutter A. Oral manifestation of COVID-19 as an inaugural symptom? J Oral Med Oral Surg 2020;26:18. Article
- 88. Spirito F, Leuci S, DI Cosola M, et al. New emerging pandemic: head and neck manifestations. Minerva Med 2022;113:905−9.ArticlePubMed
- 89. Scotto G, Fazio V, Lo Muzio E, et al. SARS-CoV-2 infection and taste alteration: an overview. Life (Basel) 2022;12:690. ArticlePubMedPMC
- 90. Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet 2020;395:1741−3.ArticlePubMedPMC
- 91. Hopkins C, Kumar N. Loss of sense of smell as marker of COVID-19 infection [Internet]. ENT UK; 2022 Jul 24 [cited 2022 Jul 24]. Available from: https://www.entuk.org/news_and_events/news/57/loss_of_sense_of_smell_as_marker_of_covid19_infection/.
- 92. Cirillo N. Taste alteration in COVID-19: a rapid review with data synthesis reveals significant geographical differences [Preprint]. Posted 2020 Sep 13. medRxiv 2020.09.11.20192831. https://doi.org/10.1101/2020.09.11.20192831.Article
- 93. UK Health Security Agency. COVID-19: investigation and initial clinical management of possible cases [Internet]. UK Health Security Agency; 2022 [cited 2022 Aug 2]. Available from: https://www.gov.uk/government/publications/wuhan-novel-coronavirus-initial-investigation-of-possible-cases/investigation-and-initial-clinical-management-of-possible-cases-of-wuhan-novel-coronavirus-wn-cov-infection.
- 94. Jafek BW, Murrow B, Michaels R, et al. Biopsies of human olfactory epithelium. Chem Senses 2002;27:623−8.ArticlePubMed
- 95. Scotto G, Fazio V, Spirito F, et al. COVID Tongue: suggestive hypothesis or clinical reality? Oral Dis 2022;28 Suppl 2:2618−9.ArticlePubMedPDF
- 96. Amorim Dos Santos J, Normando AG, Carvalho da Silva RL, et al. Oral manifestations in patients with COVID-19: a 6-month update. J Dent Res 2021;100:1321−9.ArticlePubMedPDF
- 97. Tong JY, Wong A, Zhu D, et al. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2020;163:3−11.ArticlePubMedPDF
- 98. Nijakowski K, Wyzga S, Singh N, et al. Oral manifestations in SARS-CoV-2 positive patients: a systematic review. J Clin Med 2022;11:2202. ArticlePubMedPMC
- 99. Majorana A, Bardellini E, Flocchini P, et al. Oral mucosal lesions in children from 0 to 12 years old: ten years’ experience. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010;110:e13−8.Article
- 100. Aragoneses J, Suarez A, Algar J, et al. Oral manifestations of COVID-19: updated systematic review with meta-analysis. Front Med (Lausanne) 2021;8:726753. ArticlePubMedPMC
- 101. Halboub E, Al-Maweri SA, Alanazi RH, et al. Orofacial manifestations of COVID-19: a brief review of the published literature. Braz Oral Res 2020;34:e124.ArticlePubMed
- 102. Orilisi G, Mascitti M, Togni L, et al. Oral manifestations of COVID-19 in hospitalized patients: a systematic review. Int J Environ Res Public Health 2021;18:12511. ArticlePubMedPMC
References
Figure & Data
References
Citations



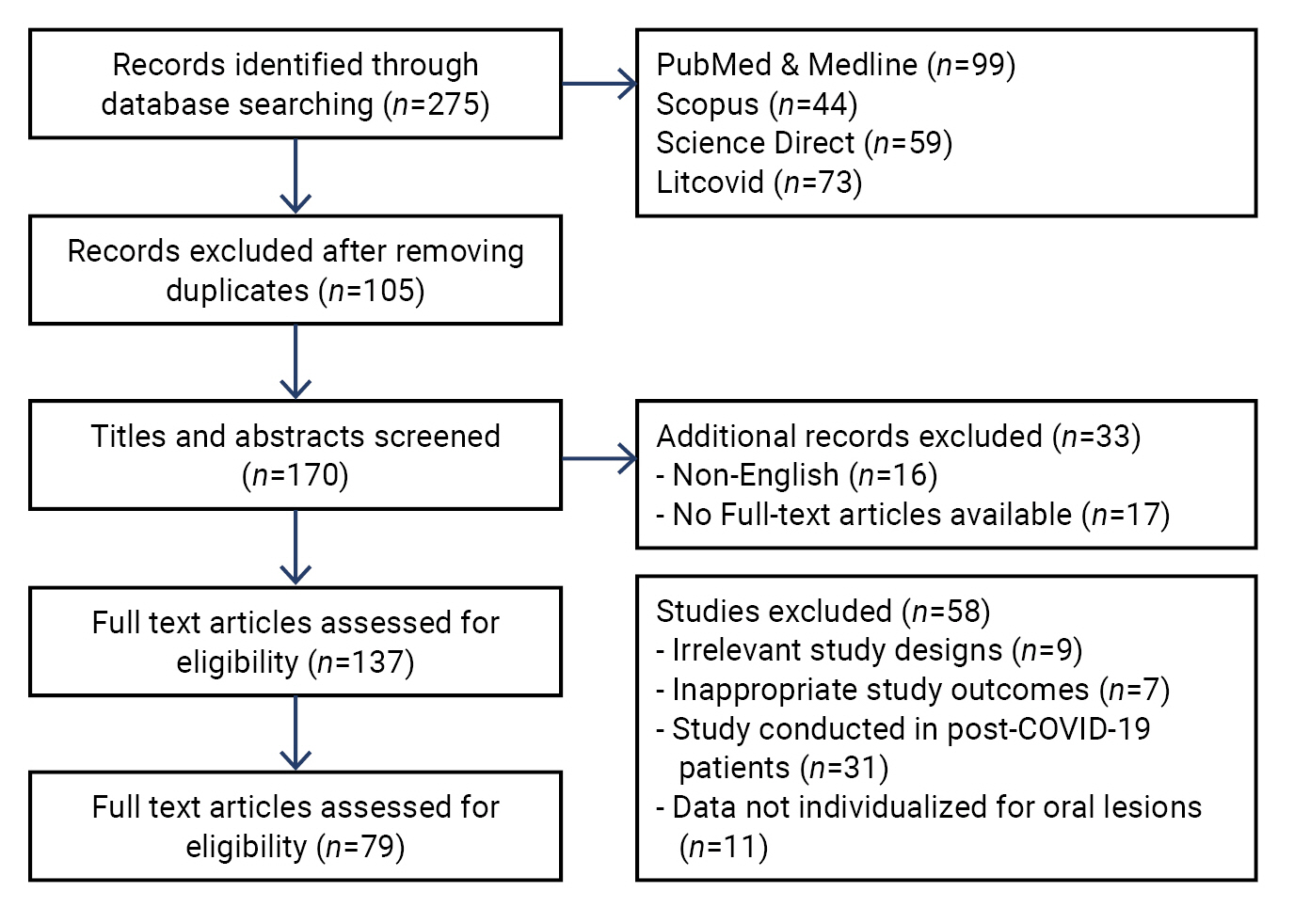
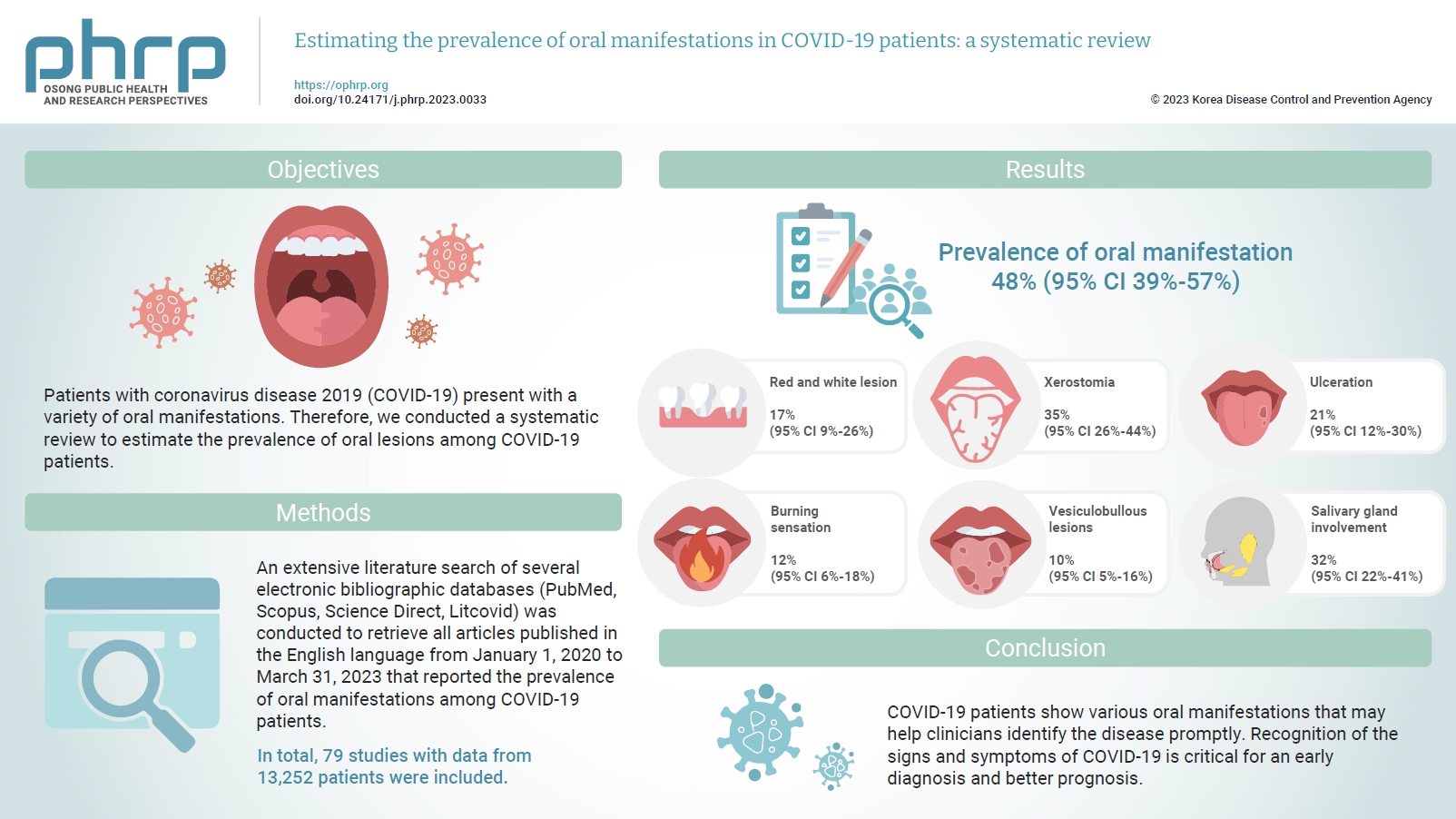
 Cite
Cite